What Happens to the Human Body in a Vacuum?
NASA / Dennis Davidson / Wikimedia Commons / Public Domain
- An Introduction to Astronomy
- Important Astronomers
- Solar System
- Stars, Planets, and Galaxies
- Space Exploration
- Weather & Climate
As humans get closer to the time when astronauts and explorers will be living and working in space for long periods of time, a lot of questions arise about what it will be like for those who make their careers "out there". There is a great deal of data based on long-duration flights by such astronauts as Mark Kelly and Peggy Whitman, but the life sciences experts at most space agencies need a lot more data to understand what will happen to future travelers. They already know that the long-term inhabitants aboard the International Space Station have experienced some major and puzzling changes to their bodies, some of which last long after they are back on Earth. Mission planners are using their experiences to help plan missions to the Moon, Mars, and beyond.
However, despite this priceless data from actual experiences, people also get a lot of non-valuable "data" from Hollywood movies about what it's like to live in space . In those cases, drama usually trumps scientific accuracy. In particular, the movies are big on gore, especially when it comes to depicting the experience of being exposed to vacuum. Unfortunately, those movies and TV shows (and video games) give the wrong impression about what it's like to be in space.

Vacuum in the Movies
In the 1981 movie "Outland," starring Sean Connery, there is a scene where a construction worker in space gets a hole in his suit. As the air leaks out, the internal pressure drops and his body is exposed to a vacuum, we watch in horror through his faceplate as he swells up and explodes. Could that really happen, or was that dramatic license?
A somewhat similar scene occurs in the 1990 Arnold Schwarzenegger movie, "Total Recall." In that movie, Schwarzenegger leaves the pressure of the habitat of a Mars colony and begins to blow up like a balloon in the much lower pressure of the Mars atmosphere, not quite a vacuum. He is saved by the creation of an entirely new atmosphere by an ancient alien machine. Again, could that happen, or was dramatic license at play?
Those scenes bring up an entirely understandable question: What happens to the human body in a vacuum? The answer is simple: it won't blow up. The blood won't boil, either. However, it will be a quick way to die if an astronaut's spacesuit is damaged.
What Really Happens in a Vacuum
There are a number of things about being in space, in a vacuum, that can cause harm to the human body. The unfortunate space traveler wouldn't be able to hold their breath for long (if at all), because it would cause lung damage. The person would probably remain conscious for several seconds until the blood without oxygen reaches the brain. Then, all bets are off.
The vacuum of space is also pretty darn cold, but the human body doesn't lose heat that fast, so a hapless astronaut would have a little time before freezing to death. It's possible that they would have some problems with their eardrums, including a rupture, but maybe not.
Being marooned in space exposes the astronaut to high radiation and the chances for a really bad sunburn. Their body might actually swell some, but not to the proportions so dramatically shown in "Total Recall." The bends are also possible, just like what happens to a diver who surfaces too quickly from a deep underwater dive. That condition is also known as "decompression sickness" and happens when dissolved gases in the bloodstream create bubbles as the person decompresses. The condition can be fatal and is taken seriously by divers, high-altitude pilots, and astronauts.
While normal blood pressure will keep a person's blood from boiling, the saliva in their mouth could very well begin to do so. There's actually evidence for that happening from an astronaut who experienced it. In 1965, while performing tests at the Johnson Space Center , a subject was accidentally exposed to a near vacuum (less than one psi) when his space suit leaked while in a vacuum chamber. He did not pass out for about fourteen seconds, by which time unoxygenated blood had reached his brain. Technicians began to repressurize the chamber within fifteen seconds and he regained consciousness at around the equivalent of 15,000 feet of altitude. He later said that his last conscious memory was of the water on his tongue beginning to boil. So, there's at least one data point about what it's like to be in a vacuum. It won't be pleasant, but it won't be like the movies, either.
There have actually been cases of parts of astronauts bodies being exposed to vacuum when suits were damaged. They survived due to quick action and safety protocols . The good news from all those experiences is that the human body is amazingly resilient. The worst problem would be lack of oxygen, not lack of pressure in the vacuum. If returned to a normal atmosphere fairly quickly, a person would survive with few if any irreversible injuries after an accidental exposure to vacuum.
More recently, astronauts on the International Space Station found an air leak from a hole made by a technician on the ground in Russia. They were in no danger of losing their air right away, but they had to go to some effort to get it plugged safely and permanently.
Edited and updated by Carolyn Collins Petersen .
- The Top Space Questions
- Where Does Space Begin?
- Can Humans Hear Sound in Space?
- Learn About the True Speed of Light and How It's Used
- How Redshift Shows the Universe is Expanding
- Radiation in Space Gives Clues about the Universe
- Time Travel: Dream or Possible Reality?
- Trace the Earliest History of Astronomy
- Reasons for Humanity to Go Back to the Moon
- Astronomy 101 - Learning About Stars
- Stargazing Through the Year
- Can We Travel Through Time to the Past?
- The Lyrid Meteor Shower: When It Occurs and How to See It
- The Composition of the Universe
- What are Rotation and Revolution?
- The Basics of Telescopes
February 14, 2008
Survival in Space Unprotected Is Possible--Briefly
But don't linger in the interstellar vacuum, or hold your breath
By Anna Gosline
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
As far as certain death in a science fiction plot line goes, being ejected into the vacuum of space is more than a pretty sure thing. A shove out of the air lock by a mutinous lieutenant or a vicious rip in a space suit, and your average movie victim is guaranteed to die quickly and quietly, though with fewer exploding body parts than screenwriters might have you believe. In reality, however, animal experiments and human accidents have shown that people can likely survive exposure to vacuum conditions for at least a couple of minutes. Not that you would remain conscious long enough to rescue yourself, but if your predicament was accidental, there could be time for fellow crew members to rescue and repressurize you with few ill effects. "In any system, there is always the possibility of equipment failure leading to injury or death. That's just the risk you run when you are in a hostile environment and you depend upon the equipment around you," says Dartmouth Medical School professor and former NASA astronaut Jay Buckey, author of the 2006 book Space Physiology . "But if you can get to someone quickly, that is good. Often spacewalks are done with two spacewalkers and there is continuous communication. So if someone is having a problem, hopefully the other can go get them and bring them in." Vacuums are indeed lethal: Under extremely low pressure air trapped in the lungs expands, tearing the tender gas-exchange tissues. This is especially grave if you are holding your breath or inhaling deeply when the pressure drops. Water in the soft tissues of your body vaporizes, causing gross swelling, though the tight seal of your skin would prevent you from actually bursting apart. Your eyes, likewise, would refrain from exploding, but continued escape of gas and water vapor leads to rapid cooling of the mouth and airways. Water and dissolved gas in the blood forms bubbles in the major veins, which travel throughout the circulatory system and block blood flow. After about one minute circulation effectively stops. The lack of oxygen to the brain renders you unconscious in less than 15 seconds, eventually killing you. "When the pressure gets very low there is just not enough oxygen. That is really the first and most important concern," Buckey says. But death is not instantaneous. For example, one 1965 study by researchers at the Brooks Air Force Base in Texas showed that dogs exposed to near vacuum—one three-hundred-eightieth of atmospheric pressure at sea level—for up to 90 seconds always survived. During their exposure, they were unconscious and paralyzed. Gas expelled from their bowels and stomachs caused simultaneous defecation, projectile vomiting and urination. They suffered massive seizures. Their tongues were often coated in ice and the dogs swelled to resemble "an inflated goatskin bag," the authors wrote. But after slight repressurization the dogs shrank back down, began to breathe, and after 10 to 15 minutes at sea level pressure, they managed to walk, though it took a few more minutes for their apparent blindness to wear off. However, dogs held at near vacuum for just a little bit longer—two full minutes or more—died frequently. If the heart was not still beating upon recompression, they could not be revived and the more rapid the decompression was, the graver the injuries no matter how much time had elapsed in the vacuum. Chimpanzees can withstand even longer exposures. In a pair of papers from NASA in 1965 and 1967, researchers found that chimpanzees could survive up to 3.5 minutes in near-vacuum conditions with no apparent cognitive defects, as measured by complex tasks months later. One chimp that was exposed for three minutes, however, showed lasting behavioral changes. Another died shortly after exposure, likely due to cardiac arrest. Although the majority of knowledge on the effects of vacuum exposure comes from animal studies, there have also been several informative—and scary—depressurization accidents involving people. For example, in 1965 a technician inside a vacuum chamber at Johnson Space Center in Houston accidentally depressurized his space suit by disrupting a hose. After 12 to 15 seconds he lost consciousness. He regained it at 27 seconds, after his suit was repressurized to about half that of sea level. The man reported that his last memory before blacking out was of the moisture on his tongue beginning to boil as well as a loss of taste sensation that lingered for four days following the accident, but he was otherwise unharmed. When it comes to exposure to the interstellar medium, you might survive it with timely help but it probably won't be to your taste.

What would happen to the human body in the vacuum of space?
Nothing pleasant.

Imagine you're an astronaut exploring the vast expanses of space and — uh oh! — you're accidentally thrown out of your spacecraft's airlock. What would happen to your body if it were exposed to the vacuum of space if you weren't wearing a spacesuit?
The first thing to note is that many Hollywood depictions of this scenario are overblown. They show people, unprotected by helmets or spacesuits, exploding or instantly freezing to death. In reality, the effects would be the same, but less exaggerated.
An astronaut floating without a suit in space wouldn't survive, but their demise would happen within minutes, not within seconds, and it would be a gnarly exit, with boiling bodily fluids and a nearly frozen nose and mouth.
Related: Why is space a vacuum?
Space is a vacuum devoid of air — meaning that, unlike on Earth , there's no atmosphere and no pressure exerted by air molecules. Atmospheric pressure determines the temperatures at which liquids boil and turn gaseous. If the pressure exerted by the air outside a liquid is high, as it is at sea level on Earth, it's harder for bubbles of gas to form, rise to the surface and escape. But because there is virtually no atmospheric pressure in space, the boiling point of liquids decreases significantly.
"As you can imagine, given that 60% of the human body is made up of water, this is a serious problem," Dr. Kris Lehnhardt, an element scientist for the Human Research Program at NASA , told Live Science. In the absence of pressure, liquid water in our bodies would boil — changing immediately from a liquid to a gas. "In essence, all of your body tissues that contain water will start to expand," he said.
Some humans have actually been exposed to near-vacuums and survived to tell the tale. In 1966, an aerospace engineer at NASA, Jim LeBlanc, was helping to test the performance of spacesuit prototypes in a massive vacuum chamber. At some point in the test, the hose feeding pressurized air into his suit was disconnected. "As I stumbled backwards, I could feel the saliva on my tongue starting to bubble just before I went unconscious, and that's kind of the last thing I remember," he recalled in the 2008 "Moon Machines" documentary series episode "The Space Suit."
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The formation of gas bubbles in bodily fluids, known as an ebullism, also occurs in deep-water scuba divers who surface too quickly because they go from an underwater environment of high pressure to low pressure at the water's surface. For suit-less astronauts, the blood flowing through the veins boils less quickly than water in the tissues because the circulatory system has its own internal pressure, but massive ebullism in the body's tissues would result rapidly. A 2013 review in the journal Aerospace Medicine and Human Performance that looked at previous exposures to vacuums in animals and humans found that they lost consciousness within 10 seconds. Some of them then lost control of their bladders and bowel systems, and the swelling in their muscles constricted blood flow to their hearts and brains , as their expanded muscles acted as a vapor lock.
— What will happen to Earth when the sun dies?
— What does it take to become an astronaut?
— Why does outer space look black?
"No human can survive this — death is likely in less than two minutes," Lehnhardt said.
According to NASA's bioastronautics data book , the vacuum of space would also pull air out of your lungs, causing you to suffocate within minutes. After an initial rush of air surged out, the vacuum would continue to pull gas and water vapor from your body through your airways. The continuous boiling of water would also produce a cooling effect — the evaporation of water molecules would absorb heat energy from your body and would cause the parts near your nose and mouth to nearly freeze. The remainder of your body would also cool, but it would do so more slowly because not as much evaporation would take place.
As astrophysicist Paul Sutter told Forbes, temperature is a measure of how much energy atoms and molecules have to move about — and because space is almost empty, there's not much to move at all, making it "cold." This also means that there isn't matter in space to transfer heat to . However, a person could freeze from the evaporation of their body's water and the slow loss of heat via the radiation emanating from their body.
The lesson from all of this? Always wear a spacesuit.
Editor's note: This story was updated at 12:45 p.m. EST on Nov. 15 to state that Dr. Kris Lehnhardt is an element scientist at NASA.
Originally published on Live Science.
Jacklin Kwan is a freelance journalist based in the United Kingdom who primarily covers science and technology stories. She graduated with a master's degree in physics from the University of Manchester, and received a Gold-Standard NCTJ diploma in Multimedia Journalism in 2021. Jacklin has written for Wired UK, Current Affairs and Science for the People.
James Webb Telescope goes 'extreme' and spots baby stars at the edge of the Milky Way (image)
Space photo of the week: Entangled galaxies form cosmic smiley face in new James Webb telescope image
2,700-year-old shields and helmet from ancient kingdom unearthed at castle in Turkey
Most Popular
- 2 James Webb Telescope goes 'extreme' and spots baby stars at the edge of the Milky Way (image)
- 3 Why can't you suffocate by holding your breath?
- 4 Space photo of the week: Entangled galaxies form cosmic smiley face in new James Webb telescope image
- 5 Did Roman gladiators really fight to the death?
A Vacuum Full of Infinite Energy

By Harnessing the Unlimited Vacuum Energy In Space, We Could Finally Reach Light Speed
Invisible vacuum energy is all around us. We could use it to power propulsion, enhance nanostructures, and build levitating devices.
Gear-obsessed editors choose every product we review. We may earn commission if you buy from a link. Why Trust Us?
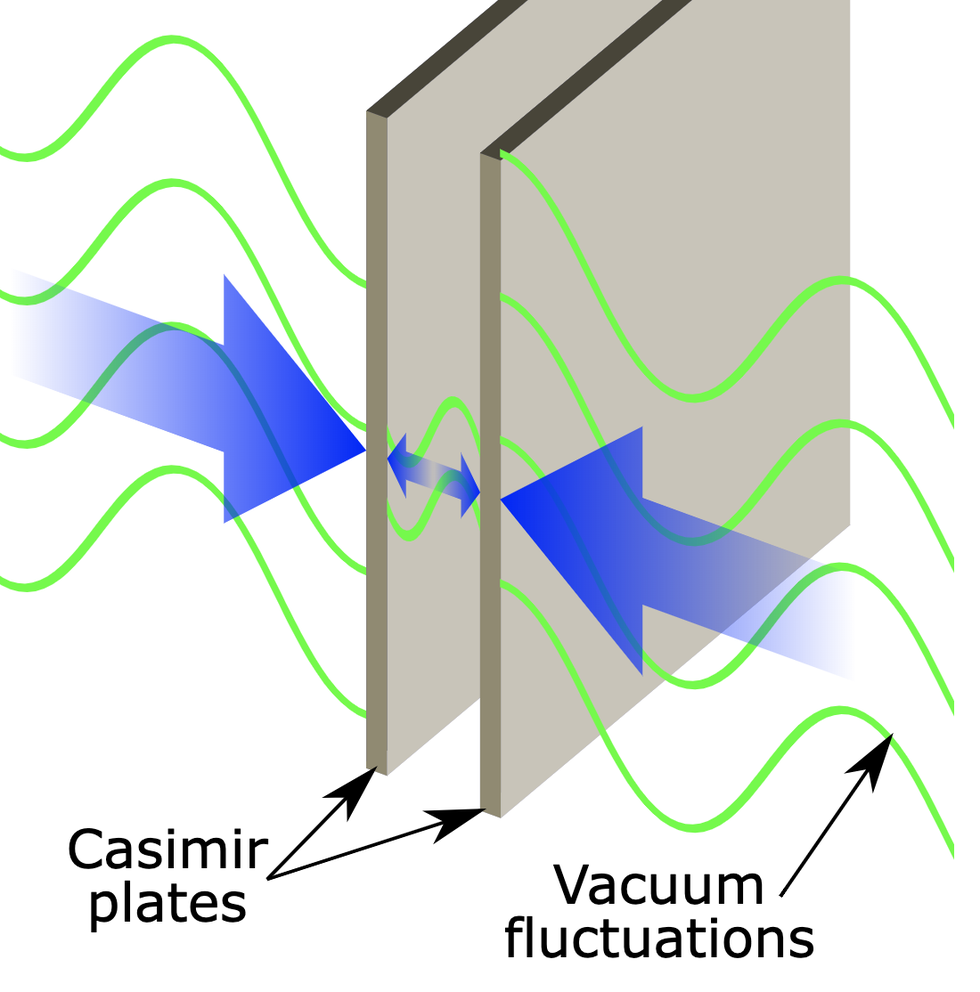
Hendrik Casimir’s idea for an experiment was simple: bring two metallic objects extremely close together and wait. Spontaneously, as if by magic, the objects will be drawn together. No external forces, no pushes or pulls, no action of gravity or tension or magnetism . The objects simply get closer. The reason? An unlimited source of vibration sitting in the very vacuum of spacetime.
This landmark experiment, first devised by Casimir just after World War II—and only realized 25 years ago—paved the way for scientists to witness the manifestations of quantum theory in a real, practical way. Quantum fields and their vibrations power our modern-day understanding of physics, from subatomic interactions to the evolution of the entire universe. And what we learned, thanks to Casimir’s work, is that infinite energy permeates the vacuum of space. There are many ideas in the science fiction universe that propose using vacuum energy to power a starship or other advanced kind of propulsion, like a warp drive. While these ideas are still dreams, the fact remains that a simple experiment, devised in 1948, set fire to our imaginations and our understanding of the universe.
Casimir , a Dutch physicist, had spent his graduate years with his advisor, Niels Bohr, one of the godfathers of quantum physics , and had picked up a liking for this new, extraordinary theory of the cosmos. But as quantum theory evolved, it started to make extremely strange statements about the universe . The quantum world is weird , and its ultimate weirdness is normally invisible to us, operating at scales well below our normal human perception or experimentation. Casimir started to wonder how we might be able to test those ideas.
He went on to discover a clever way to measure the effects of ever-present infinite quantum fields merely using bits of metal held extremely close together. His work showed that quantum behavior can manifest in surprising ways that we can measure. It also showed that the strangeness of quantum behavior is real and can’t be ignored, and what quantum mechanics says about the workings of the universe —no matter how bizarre—must be believed.
Quantum Fields Are Otherworldly, But Very Real
One of the lessons of the quantum world is that particles , like electrons, photons, neutrinos, and whatnot, aren’t what they seem to be. Instead, each of the particles that we see in nature is actually just a piece of a much larger, grander entity. These grander entities are known as quantum fields, and the fields soak every bit of space and time—all throughout the universe—the same way that oil and vinegar soaks a piece of bread.

There is a quantum field for every kind of particle: one field for the electrons , one for the photons, and so on. These fields are invisible to us, but they make up the fundamental building blocks of existence. They are constantly vibrating and buzzing. When the fields vibrate with enough energy, particles appear. When the fields die down, the particles disappear. Another way to look at this is to say that what we call a “particle” is really a localized vibration of a quantum field. When two particles interact, it’s really just two pieces of quantum fields interacting with each other.
There’s no such thing as a true vacuum; wherever you go, there are always vibrating quantum fields.
These quantum fields are always vibrating, even when those vibrations aren’t strong enough to produce a particle. If you take a box and empty out all of the stuff—all the electrons, all the photons, all the neutrinos, all the everything—the box is still filled with these quantum fields. Since those fields vibrate even in isolation, that means the box is filled with invisible vacuum energy, also known as zero-point energy—the energy of these fundamental vibrations.
In fact, you can calculate how many vibrations are in each of these quantum fields ... and the answer is infinity! There are small ones, medium ones, big ones, and gigantic ones, all flopping on top of each other continuously, as if spacetime itself was boiling at the subatomic level. This means that the vacuum of the universe really is made of something. There’s no such thing as a true vacuum; wherever you go, there are always vibrating quantum fields.
A Simple Experiment Involves Multiple Infinities
This is where Casimir’s experiment comes in: If you take two metal plates and stick them really, really close together, the quantum fields between those plates must behave in a certain way: the wavelengths of their vibrations must fit perfectly between the plates, just like the vibrations on a guitar string have to fit their wavelengths to the length of the string. In the quantum case, there are still an infinite number of vibrations between the plates, but—and this is crucial—there are not as many infinite vibrations between the plates as there are outside the plates.
How does this make sense? In mathematics, not all infinities are the same, and we’ve developed clever tools to be able to compare them. For example, consider one kind of infinity where you add successive numbers to each other. You start with 1, then add 2, then add 3, then add 4, and so on. If you keep that addition going forever, you’ll reach infinity. Now consider another kind of addition, this one involving powers of 10. You start with 101, then add to it 102, then 103, then 104, and keep going.
Again, if you keep this series going on forever, you’ll also reach infinity. But in a sense you’ll “get” to infinity faster. So by carefully subtracting these two sequences, you can get a measure of their difference even though they both go to infinity.
Using this clever bit of mathematics, we can subtract the two kinds of infinities—the ones between the metal plates and the ones outside—and arrive at a finite number. This means that there really are more quantum vibrations outside the two plates than there are inside the plates. This phenomenon leads to the conclusion that the quantum fields outside the plates push the two plates together, something called the Casimir effect in Hendrik’s honor.
The effect is incredibly small, roughly 10 -12 Newtons, and it requires the metal plates to be within a micrometer of each other. (One Newton is the force which accelerates an object of 1 kilogram by 1 meter per second squared.) So, even though Casimir could predict the existence of this quantum effect, it wasn’t until 1997 that we were finally able to measure it, thanks to the efforts of Yale physicist Steve Lamoreaux.
🦎 Quantum Physics In Action Perhaps most strangely, the creature with the deepest connection to the fundamental quantum nature of the universe is the gecko. Geckos have the ability to walk on walls, and even upside-down on ceilings. To accomplish this feat, a gecko’s limbs are covered in countless, microscopic hair-like fibers. These fibers get close enough to the molecules of the surface it wants to climb on for the Casimir effect to take action. It creates an attractive force between the hair and the surface. Each individual hair provides only an extremely tiny amount of force, but all the hairs combined are enough to support the gecko.
In this experimental setup, which can fit on a kitchen countertop, the plates don’t magically pull themselves together. Instead it’s the infinite vibrating quantum fields of spacetime pushing them together from the outside.
We don’t normally see or sense or experience the Casimir effect. But when we want to design micro- and nano-scale machines , we have to account for these additional forces. For example, researchers have designed micro-scale sensors that can monitor the flow of chemicals on a molecule-by-molecule basis, but the Casimir effect can disrupt the operations of this sensor if we didn’t know about it.
Scientists Are Exploring the Potential of Vacuum Energy
For several years, researchers have been investigating the possibility that we really can extract vacuum energy and use it for energy. A 2002 patent was awarded for a device that captures the electric charge from the Casimir experimental setup’s two metal plates, charging a storage battery. The device can be used as a generator. “To continuously generate power a plurality of metal plates are fixed around a core and rotated like a gyrocompass,” according to the patent.
💫 Scientists Believe Light Speed Travel Is Possible. Here’s How.
The U.S. Defense Department’s Defense Advanced Research Projects Agency (DARPA) gave researchers $10 million in 2009 to pursue a better understanding of the Casimir force. Though progress in actually using vacuum energy continues to be incremental, this line of energy research could give rise to innovations in nanotechnology, such as building a device capable of levitation, researchers said at the time.
At the University of Colorado in Boulder, Garret Moddel ’s research group has developed devices that produce power “that appears to result from zero-point energy quantum fluctuations,” according to the group’s website . Their device essentially recreates Casimir’s experiment, generating an electrical current between the two metal layers that researchers could measure, despite applying no electrical voltage.
As for Casimir himself, who was immersed in a quantum revolution unfolding at Leiden University, he had a tendency to downplay the importance of his own work. In his autobiography, Haphazard Reality , Casimir said, “The story of my own life is of no particular interest.” And his monumental 1948 paper designing his experiment ends with the simple statement, “Although the effect is small, an experimental confirmation seems not infeasable and might be of a certain interest.”
In fact, his initial insight did not make a big splash on the scientific community, nor were there glowing popular press accounts of his experiment. Part of the reason was Casimir’s own modesty, and another is that he soon left academic research to pursue a career in industry. But despite these humble beginnings, his work cannot be understated.
Today, we continue to refine Casimir’s original experimental setup, searching for any cracks in our theories, and we use it as a foundation to explore ever more deeply the fundamental nature of the cosmos.
Paul M. Sutter is a science educator and a theoretical cosmologist at the Institute for Advanced Computational Science at Stony Brook University and the author of How to Die in Space: A Journey Through Dangerous Astrophysical Phenomena and Your Place in the Universe: Understanding Our Big, Messy Existence. Sutter is also the host of various science programs, and he’s on social media. Check out his Ask a Spaceman podcast and his YouTube page .
Pop Mech Pro: Science

Aliens May Be Hiding Underground, Scientists Say

The Brain Could Be 100 Million Times More Powerful

Our Consciousness May Come From a Higher Dimension

Could This Metal Shard Be Alien Technology?

Wormholes May Be a Portal for Interstellar Travel

Archaeologists Find an Ancient Immortality Potion

We’re Plugging Into the World’s Biggest Battery

Do Psychedelics Reveal an ‘Ultimate Reality?’

Fourth-Dimensional Aliens Could Be Spying On Us

Aliens May Possess a Form of Consciousness

Japan Is the World’s Top Hotspot for UFO Sightings

Are Underwater UFOs an Imminent Threat?
by Michael Barratt
Today, the body at vacuum. The University of Houston presents this series about the machines that make our civilization run, and the people whose ingenuity created them.
I n a famous scene in Stanley Kubrick's "2001 A Space Odyssey", astronaut Dave Bowman ejects himself through open space into an unpressurized airlock of the mother ship — this after the HAL 9000 computer refuses to let him in. After a couple of bounces, he manages to close the outer hatch and pressurize the airlock. A desperate survival move, but is it plausible? Hollywood and science fiction vary considerably in depicting the body in open space, from almost negligible effects to messy whole body explosions. Here's some reality.

The vapor pressure of water at body temperature is about one 16th of atmospheric pressure. Below this pressure, which you find above an altitude of about 63,000 ft, body fluids begin to boil away. Moist surfaces experience this immediately, such as the eye, mouth and throat, and airways. Deeper inside, body water in low pressure areas also turns to its gas phase, water vapor. This occurs rapidly in the lung and beneath the skin. Bubbles of water vapor also form in venous blood; these essentially vapor lock the circulation. We call the syndrome associated with the formation of water vapor bubbles in the body at vacuum ebullism . But there is a little more to it.
Because the lungs communicate freely with the outside, a sudden pressure drop causes the air in the lungs to rapidly expand, seeking an outlet. Keeping an open airway ensures that the air rushes outward, better than the alternative of trying to hold it in and causing a traumatic rupture of the lung. But of course respiration is no longer possible. With no circulation and no air to provide oxygen anyway, about 12 seconds of useful consciousness is available.
In human spaceflight, this is a constant threat. In 1971, the Soyuz 11 spacecraft tragically depressurized on entry at over 96 miles; the three unsuited occupants perished.

But there are at least a couple of human exposures to whole body vacuum that ended happily. In 1966, a technician testing a space suit in a vacuum chamber experienced a rapid loss of suit pressure due to equipment failure. He recalled the sensation of saliva boiling off his tongue before losing consciousness. The chamber was rapidly repressurized, he regained consciousness quickly, and went home for lunch. Another man was accidentally exposed to vacuum in an industrial chamber; it was at least three minutes before he was repressurized. He required intensive medical care, but eventually regained full function. These instances show that ebullism is not inevitably fatal — and the body holds together just fine.
And so we return to Dave Bowman's predicament. From my rough timing of the movie's events, it was about 8 sec between explosive decompression and activating the handle to repress the airlock, then an additional 4 ' 5 seconds to close the door and begin repressurizing. Could he have managed the feat? The answer is probably. With the presence of mind to exhale and keep an open airway, he may have performed this necessary task without injury. But his was a unique scenario of planned sudden decompression with a pressurized endpoint.
So if you should ever face decompression — well, don't hold your breath.
I'm NASA astronaut Michael Barratt for the University of Houston, where we're interested in the way inventive minds work.
End Notes :
Aristotle stated in the 4th century BC that 'nature abhors a vacuum'. As has been realized since then however, there is an awful lot of it out there. Away from our planet's surface where the atmosphere is nice and dense, the environment is summarily inhospitable. In a prior episode we discussed the threshold altitude where the surrounding pressure is roughly equal to the vapor pressure of water at body temperature; this is about 63,000 feet, an altitude known as Armstong's Line. Said another way, the boiling point of water at this altitude is about 98.6 degrees Fahrenheit. As such, for human exposure, you add ebullism to the already present conditions of hypoxia , not enough oxygen, and decompression sickness , similar to what a scuba diver might get if he does not rise slowly enough to let the excess nitrogen out of the body.
The issue of whether Dave Bowman could have done what he did was a favorite discussion topic in my aerospace medical training class at Wright State University many years ago. It forces the consideration of many factors central to human physiology in extreme environments — fundamental among them the need for pressure and oxygen. But all indications are that Stanley Kubrik and his staff did their homework. By the time this movie was made, there had been several studies by the USAF using humans for partial body exposure and animals for whole body exposure to short term vacuum. And of course there was the experience of Jim Leblanc, the suit technician described in the chamber incident in 1966. A remarkable video of this event is shown at this site: http://vodpod.com/watch/3935109-nasas-jim-leblanc-survives-life-threatening-space-vacuum-accidentvideo .
The term ebullism , from the Latin ebullire, to boil up, was first suggested by Captain Julian Ward in a thoughtful treatise in 1956. This was offered as an alternative to simply 'boiling' of body fluids, and is still the preferred term today.
An interesting occurrence of partial ebullism occurred during a high altitude balloon flight, in which Joseph Kittinger experienced a glove pressurization failure while ascending well above the 63,000 feet where ebullism occurs. His hand was described as swelling to about twice normal size and was quite painful. He jumped from a height of over 102,000 feet, free falling for over four minutes and eventually opening a parachute at 18,000 feet. While falling back into the atmosphere, his hand repressurized and returned to normal size and full function.

In the case of explosive decompression, there are of course other hazards and events. Whatever water vapor is in the air instantly condenses due to the rapid temperature drop. Some of us experience this during chamber training, where we undergo a rapid decompression from simulated altitudes of say 8000 feet to over 20,000; this is well below Armstrong's line but it definitely gets your attention when some of the air rushes out of your lungs and fog instantly appears. This was also shown in the 2001 scene. The fog rapidly dissipates as the water turns back into vapor in the surrounding vacuum. For the case of decompressing explosively to complete vacuum, it is possible that even keeping an open airway (mouth open, glottis open) may not present a big enough pipe to allow the air to escape the lungs with an injury causing pressure buildup. In addition, whatever gas may be present in the stomach will instantly expand, and may induce vomiting by forcing stomach contents back up the esophagus as a path of escape. Overall, such events are good to avoid.

Joseph Kittinger in his full pressure suit, preparing for ascent well above the threshold of physiologic vacuum in the US Air Force Man High program.
I was fortunate enough to perform a couple of space walks during my tour on the International Space Station, both in the Russian Orlan space suit. One cannot avoid thinking about the possibility of suit depressurization, and we design and train to avoid this to the extent possible. And while we do not have a ship's computer that controls our vital functions, I did feel some comfort in knowing that we left our airlock hatch wide open.

The Russian Orlan space suit I used for two spacewalks while onboard the International Space Station. This is pressurized to just under 6 pounds per square inch (psi), or about four tenths of an atmosphere. Photo courtesy NASA .
References:
Julian Ward. The True Nature of the Boiling of Body Fluids in Space. Journal of Aviation Medicine, October 1956 27(5) pp. 429-439.
This is one the landmark articles in the aerospace medical literature by one of our pioneers. Captain Ward was tragically lost in an aircraft accident. To honor his memory, the Society of US Air Force Flight Surgeons annually bestows the Julian Ward award for advances in aerospace medicine.
Norfleet, WT. Decompression-Related Disorders: Decompression Sickness, Arterial Gas Embolism, and Ebullism Syndrome Chapter 11. Principles of Clinical Medicine for Space Flight. Michael Barratt and Sam Pool, Eds. Springer-Verlag, 2008.
For those looking for the technical details, Dr. Norfleet wrote a thorough treatise of state-of -the-art understanding of ebullism in our textbook.
Kolesari GL, EP Kindwall, "Survival Following Accidental Decompression to an Altitude Greater Than 74,000 Feet (22,555 m)," Aviation, Space and Environmental Medicine , Dec. 1982, 53(12):1211-1214.
Medical case report of the gentlemen mentioned in the industrial vacuum chamber incident.
Roth EM, "Rapid (Explosive) Decompression Emergencies in Pressure-Suited Subjects," NASA CR-1223, 1968.
This is a NASA technical report that describes the details of the suit technician's mishap, along with a discussion of what was known of ebullism and rapid decompression.
The Russian Orlan space suit photograph is courtesy of NASA.
The remaining images are from Wikipedia. The "2001: A Space Odyssey" movie image is from Wikipedia and falls under fair use act because of quality and use.
Note added by John Lienhard on Aug. 21, 2108: I have just had occasion to talk with Hank Rotter, a NASA engineer since 1963. He mentioned that he'd worked in vacuum chambers back in the Apollo spacecraft era. So I told him about this episode and the 1966 accident. "I was there when that happened," he replied. It was he who opened the door to the chamber and cut the malfunctioning backpack. He also took pains to say that the real hero of the day was a forgotten technician named R. L. Clay who had the presence of mind to repressurize the chamber immediately when the space suit's backpack malfunctioned. It still took another 87 seconds, but his action saved a life.
The man in the space suit was NASA engineer James LeBlanc who went on to become a Division Chief. Here is a video of the event . In it, you'll see Hank Rotter entering the chamber after the accident. Rotter also remarked that he was perfectly calm when it happened, but that he was seriously shaken in the days that followed. Once he thought about it, the near death of LeBlanc was horrifying.
- Engines Transcripts
- Search Episodes by Keyword
- Airing schedule for HPM

Suggested Searches
- Climate Change
- Expedition 64
- Mars perseverance
- SpaceX Crew-2
- International Space Station
- View All Topics A-Z
Humans in Space
Earth & climate, the solar system, the universe, aeronautics, learning resources, news & events.
Arctic Sea Ice Near Historic Low; Antarctic Ice Continues Decline

Hubble Lights the Way with New Multiwavelength Galaxy View

What’s Up: September 2024 Skywatching Tips from NASA
- Search All NASA Missions
- A to Z List of Missions
- Upcoming Launches and Landings
- Spaceships and Rockets
- Communicating with Missions
- James Webb Space Telescope
- Hubble Space Telescope
- Why Go to Space
- Commercial Space
- Destinations
- Living in Space
- Explore Earth Science
- Earth, Our Planet
- Earth Science in Action
- Earth Multimedia
- Earth Science Researchers
- Pluto & Dwarf Planets
- Asteroids, Comets & Meteors
- The Kuiper Belt
- The Oort Cloud
- Skywatching
- The Search for Life in the Universe
- Black Holes
- The Big Bang
- Dark Energy & Dark Matter
- Earth Science
- Planetary Science
- Astrophysics & Space Science
- The Sun & Heliophysics
- Biological & Physical Sciences
- Lunar Science
- Citizen Science
- Astromaterials
- Aeronautics Research
- Human Space Travel Research
- Science in the Air
- NASA Aircraft
- Flight Innovation
- Supersonic Flight
- Air Traffic Solutions
- Green Aviation Tech
- Drones & You
- Technology Transfer & Spinoffs
- Space Travel Technology
- Technology Living in Space
- Manufacturing and Materials
- Science Instruments
- For Kids and Students
- For Educators
- For Colleges and Universities
- For Professionals
- Science for Everyone
- Requests for Exhibits, Artifacts, or Speakers
- STEM Engagement at NASA
- NASA's Impacts
- Centers and Facilities
- Directorates
- Organizations
- People of NASA
- Internships
- Our History
- Doing Business with NASA
- Get Involved
NASA en Español
- Aeronáutica
- Ciencias Terrestres
- Sistema Solar
- All NASA News
- Video Series on NASA+
- Newsletters
- Social Media
- Media Resources
- Upcoming Launches & Landings
- Virtual Guest Program
- Image of the Day
- Sounds and Ringtones
- Interactives
- STEM Multimedia
New Video Series Spotlights Engineers on NASA’s Europa Clipper Mission

Celebrating 10 Years at Mars with NASA’s MAVEN Mission

What You Need to Know about NASA’s SpaceX Crew-9 Mission
Educational activities in space.

NASA Astronaut Tracy C. Dyson’s Scientific Mission aboard Space Station

Station Science Top News: September 13, 2024

NASA’s Eyes for Museums

NASA Analysis Shows Irreversible Sea Level Rise for Pacific Islands

Oct. 2 Annular Solar Eclipse

In Odd Galaxy, NASA’s Webb Finds Potential Missing Link to First Stars

2024 SARP West Closeout

Girls in STEM Inspired to Fly High at NASA Kennedy

ARMD Solicitations

Students Soar at NASA Glenn’s Aviation Day
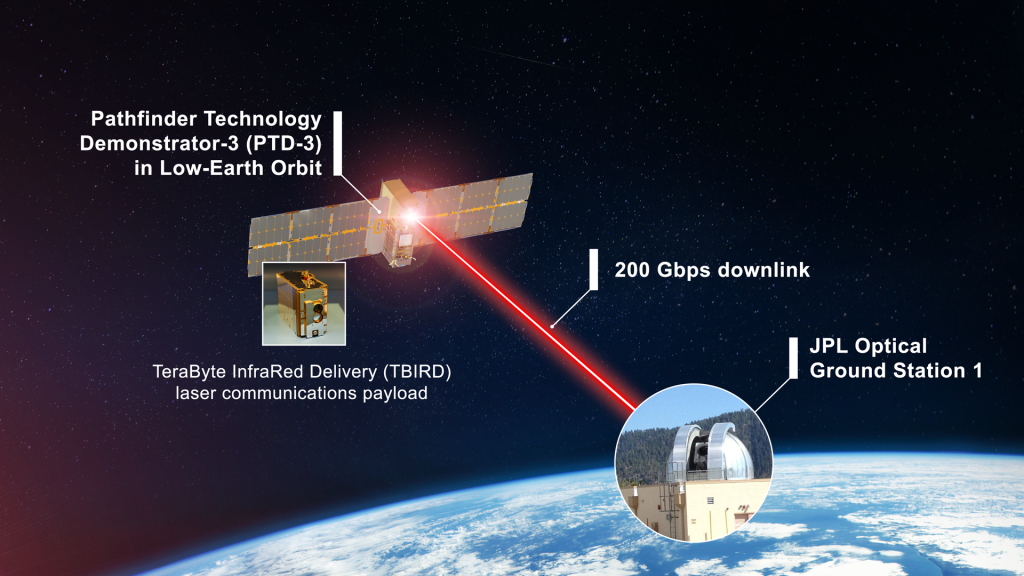
NASA’s Record-Breaking Laser Demo Completes Mission

Amendment 52: B.9 Low-Cost Access to Space 2028 Peruvian campaign Update

Amendment 51: F.13 Lunar Terrain Vehicle Instruments Program Final Text and Due Dates.

NASA Moon to Mars Architecture Art Challenge

Bring NASA Into Your Classroom This Fall Through Virtual Experiences

How Do I Navigate NASA Learning Resources and Opportunities?

NASA Helps Build New Federal Sea Level Rise Website

NASA Expands Small Business, Industry Engagement Resources

La NASA invita a los medios al lanzamiento de Europa Clipper

El X-59 de la NASA avanza en las pruebas de preparación para volar

La NASA invita a creadores de las redes sociales al lanzamiento de la misión Europa Clipper
Thermal vacuum.
Introduction
Human-rated testing, thermal vacuum testing, dust chambers, thermal testing.
Thermal Vacuum testing is critical for designing spaceflight hardware to ensure its resilience and functionality in the extreme environment variations encountered in outer space. Johnson Space Center (JSC) Thermal Vacuum Test Facilities provide thermal vacuum chamber test operations for both crewed and uncrewed test environments. The facilities offer a wide range of performance capability, which can be matched to the individual test requirements of smaller test articles or large test article components and subsystems. JSC offers lunar environmental test capabilities for subsystem and system hardware and assemblies. This capability complements existing Agency resources for both ambient dust testing and dirty thermal vacuum capability. Typical uses of these chambers have included development, engineering evaluation, and qualification testing of spacecraft components, subassemblies, and experiments; and preflight thermal-vacuum conditioning of flight hardware.
JSC is also home to the Radiant Heat Test Facility, originally constructed to perform development and certification tests for the Space Shuttle thermal protection system (TPS). Two test chambers are equipped with vacuum pumps and heater assemblies to produce a variable pressure profile and heat fluxes for simulating atmospheric re-entry conditions. We invite customers to leverage our diverse range of thermal testing capabilities in order to pursue our collective exploration of the solar system.
JSC Chamber B
Overview | Chamber B is used for human testing in a vacuum environment and for crewed space operations testing.
Details | NASA JSC Chamber B provides space environmental testing with vacuum thermal conditions. Chamber B is a human-rated chamber equipped with a traversing monorail that provides weight relief to one suited crew member at a time. The chamber also has dual crew airlocks to provide easy access to the test articles as well as a means of transporting test crew members to the test environment and back during tests. Chamber B has an internal volume of 7.6 m (25 ft) diameter x 7.9 m (26 ft). Its usable test volume and high-fidelity space simulation capabilities are adaptable for thermal vacuum testing of a wide variety of test articles. The low temperature range of the chamber is -300° F. The pressure range of the chamber is from 1×10^-6 torr to 760 torr.
Thermal-Vacuum Human-Rated Testing
Overview | Johnson Space Center (JSC) is the world leader in human-rated testing in a simulated space environment, offering human testing in vacuum, thermal– vacuum, and vibration environments. JSC offers a collection of unique knowledge and experience as to what works well within the hostile environment of space and what does not. This knowledge is available to support crewed spacecraft and space system hardware design reviews and flight– like simulation of Extravehicular Activity (EVA) operations in pressures ranging from vacuum to one atmosphere.
- Human-rated hardware testing in vacuum and thermal–vacuum environments
- Space suit development testing
- Flight crew training
- Environmental control and life support system testing
- Metabolic loading to life support systems
- Parametric testing
- Emergency and mobility accommodations of suited crew person
Human-in-the-loop air revitalization system testing
- Carbon dioxide (CO2) removal or reduction testing
- Oxygen (O2) generation testing
- Trace contaminant control testing
Human-rated vibration testing
- Thermal analysis, including human thermal modeling
JSC Chamber A
Overview | Chamber A is the largest of the thermal-vacuum test facilities at JSC. The chamber’s usable test volume and high-fidelity space simulation capabilities are adaptable for thermal-vacuum testing of a wide variety of test articles, including entire space vehicles.
Details | NASA JSC Chamber A provides a high vacuum thermal chamber with a usable test volume and space simulation capabilities that are adaptable for testing entire space vehicles. The chamber can simulate low temperatures of deep space (35K) within a 55 feet diameter by 80 feet tall shroud volume. The pressure range of the chamber is from 1×10^-6 torr to 760 torr. Additional test support equipment includes mass spectrometers, infrared cameras, and television cameras. The numerous flanges at all levels provide ample pass-throughs for electrical, instrumentation, and gasses to support large systems.
Specialized Chambers and Environmental Test Complex
Overview | Contains a collection of chambers of varying thermal & thermal/vacuum environment capabilities configured for testing hardware ranging from flight (qualification) to development hardware in support of JSC Government Furnished Equipment (GFE) activities.
- Temperature and humidity cycling
- Accurate determination of design factors
- Operating temperatures
- Changes in absorptive or emissive properties of thermal coating
- Changes in electrical or mechanical properties of materials
- Accelerated electrical or electronic components burn-ins and life-cycle testing
- Environmental cycling (thermal and humidity) for materials survivability
Thermal Vacuum Testing
Overview | Johnson Space Center (JSC) Thermal Vacuum Test Facilities provide thermal vacuum chamber test operations for both manned and unmanned test environments. The facilities offer a wide range of performance capability, which can be matched to the individual test requirements of smaller test articles or large test article components and subsystems. Typical uses of these chambers have included development, engineering evaluation, and qualification testing of spacecraft components, subassemblies, and experiments; preflight thermal– vacuum conditioning of flight hardware; development and calibration of instruments for use in the large chambers or in– flight; spacecraft seal studies; photographic film emulsion studies; and optical surface contamination studies.
Details | Human-rated space environmental testing
- 12.5-foot internal diameter spherical chamber with ~78-inch diameter clear entry for easy access
- Materials outgassing evaluations
- Accelerated electrical and electronic component burn-ins and life-cycle testing
- Materials and hardware testing in extreme environments (manned and unmanned)
- Determination of design factors
- Combined thermal and pressure-load distortions of dimensionally critical structural elements
- Fluid and gas leak rates
- Evolution of harmful or undesirable offgassing products
- Presence of conditions conducive to electrical arc or corona discharge
RITF Thermal Vacuum Chambers
Overview | Thermal Vacuum chambers are used to test or prove out a design prior to flight to ensure its reliability. The Receiving, Inspection and Test Facility (RITF) team operates two TVAC chambers capable of housing units approximately 24” X 24”.
Details | The purpose of the RITF TVAC chambers are to expose payloads, mechanisms, or components to representative space environment conditions – a vacuum state combined with repeated cycling between high and low thermal extremes to assess their likely flight performance. Heating and cooling of components and devices can be investigated using the chamber.
- Vacuum Range: Up to 5×10 -6 torr
- Temperature range: -100°C to + 250°C
- 24″ x 24″ internal space
15-foot Dirty Thermal Vacuum Chamber
Overview | NASA JSC 15-foot dirty thermal vacuum chamber (TVAC) provides unique testing capabilities for dust and planetary surface environments. The 15-foot chamber is a spherical chamber designed to test advanced concepts, especially for battery power systems, space vehicle actuators and auxiliary power units.
- Vacuum conditions: 1×10^-6 torr to 760 torr
- Thermal conditions: -186°C to +120°C
- Air, GN2 pressurization
- Feed-throughs for high-power electrical connections and high-channel count data
- Control automation enabling low-cost operations
- Ambient dust containment room for regolith control and testing
- Hardware exposure testing to dust / regolith
- Regolith bin for design and test of excavation, processing, or construction technology
3-foot Dirty Thermal Vacuum Chamber
Overview | NASA JSC’s 3-foot dirty thermal vacuum chamber (TVAC) provides unique testing capabilities for dust and planetary surface environments. The 3-foot chamber is a 3ft x 3ft x 3ft cube designed to test component level concepts in a lunar simulated environment.
- Thermal Vacuum capability with dust mitigation for pumping system
- 36”x36”x36” (0.9m x 0.9m x 0.9m) cube shape
- Rough or High vacuum (10^-5 torr range quickly, 10^-7 torr demonstrated)
- Shroud temperature range of -300°F to +300°F (-185°C to +148°C)
- Shroud walls independently controlled for zone temperature conditioning if needed
Regolith Simulant Component Test Lab
Overview | The Component Test Lab offers multiple small vacuum environments for testing in simulant and a simulant dispersal test box with ambient dispersal capabilities.
- Multiple 1’-3’ thermal vacuum bell jars for testing with simulant
- Ambient 2’ x 2’ x 3’ dust box for quick and easy functional testing with fan-blown simulant
- Capable of reaching 0.05 torr (ultimate)
- Air or GN2 repress
- Dimensions:
- Chamber: 1.1m x 0.74m x 0.9m (44” x 29” x 35”)
- Airlock: ⌀0.36m x 0.41m (⌀14” x 16”)
- SAE J575 Section 4.5
- DIN 40050, Part 9, IP Codes 5K and 6K
- ISO 20653, Section 8.3.1
- IEC 529, IP Codes 5 & 6
- 72”x36”x30” (1.8m x 0.9m x 0.76m) interior
- Max test article weight: 50 lbs (22.7 kg)
- Timed dusting allows for repeated application of dust to moving test articles
- Validated for use with lunar simulants
Thermal Testing
Overview | Johnson Space Center (JSC) thermal test facilities offer a wide range of performance capability, which can be matched to the individual test requirements of smaller test articles or large test article components and subsystems. Typical uses of these chambers have included development, engineering evaluation, and qualification testing of spacecraft components, subassemblies and experiments, and preflight thermal conditioning of flight hardware.
Details | Temperature and humidity cycling
- Changes in electrical or mechanical properties of materials
- Accelerated electrical or electronic components burn-ins and life-cycle testing
- Battery performance and abuse testing
- Subscale evaluation of thermal components
Radiant Heating Test Facility (RHTF)
Overview | The Radiant Heat Test Facility (RHTF) provides for simulation of the heating experienced by spacecraft as they enter planetary atmospheres. The facility provides the capability to perform multi-zone, high-temperature, radiant heat testing of large spacecraft thermal protection systems and associated structures in a controlled pressure environment to simulate entry thermal profiles, thermal gradients, and pressures.
- Basic material testing and screening
- Development testing – gaps, seals, and attachments
- Clipped hardware
- RCS nozzles, antennas, instrument penetration, windows, and hatches
- Sustaining Engineering
- Orbital debris
- Design changes
- Recertification of materials
- Ascent heating and pressure decay
- On-orbit cold soak
- Reentry heating and pressure
- Uniquely shaped large scale system tests
- Wing leading edge
- Small scale tests
- Materials screening
- Conductivity testing
- Advanced materials
Thermal Conditioning (Burn-in)
Overview | The Receiving, Inspection and Test Facility (RITF) team maintains multiple temperature and humidity chambers to provide testing support of electronics, composites, and other materials that a customer may need screened. Burn-in is the process by which components of a system are exercised prior to being placed in service (and often, prior to the system being completely assembled from those components).
Details | Burn-in is a test performed for the purpose of screening or eliminating marginal devices, those with inherent defects or defects resulting from manufacturing aberrations which cause time and stress dependent failures. The combination of variable humidity and temperature are a particular concern for the design of electronics, where moisture build up may lead to short circuiting and product failure. Similarly, high, and low humidity can affect electrical characteristics such as increased conductivity at higher humidity or electrostatic discharges at lower humidity. This screening capability can be used to identify design faults/problems that if corrected in the early stages of the design phase will save rework later.
- Thermal Conditioning (Burn -In) Temperature Range: -100 degC to +300 degC
- Thermal Cycling Range from -100 degC to +125 degC
- Humidity Cycling Range from +250 degC to +850 degC @ 20% RH to 85% RH (Relative Humidity)

Related Capabilities

Connect With Us
Whether you are a public agency, private company, or academic institution, we look forward to hearing from you.
What Happens To Humans When Exposed To The Vacuum Of Space?
Asphyxiation, depressurization.
If you are exposed to the vacuum of space, the first thing that will happen is asphyxiation, or lack of oxygen in the bloodstream. You will lose consciousness after about two minutes, and your body will start to swell due to the loss of atmospheric pressure. You will also freeze and be exposed to radiation from the sun and other cosmic bodies. If you are not brought back into a pressurized and protected space, your body will be floating in space for a very long time.
When you were a child, perhaps you looked up at the sky from time to time. You looked up, saw the night sky, and for better or worse, you started wondering. Maybe that prompted you to dream of becoming an astronaut somewhere down the line, drifting through the vast expanse, the cold darkness, and the infinity of nothingness. It’s a common dream, but it feels a bit terrifying, doesn’t it?
Every person that has ever gone into space has always had the same reservations at one time or another. Maybe it was during their training for the long journey, or in the moments strapping up for the launch before a humongous amount of fuel ejected them into the orbit, or even when they first laid eyes on a shooting star.

Beautiful. Magnificient. Lethal. Those are just a few descriptions of what outer space truly is. Therefore, if you ever wondered what would happen if you were actually stranded in space without a suit, gear, or anything else between your outstretched hand and the absolute vacuum, then this article is for you!
Time and again, you have seen in movies, read in novels, or heard it from someone’s imaginary account in which the hull of a spaceship explodes and humans fly out without any equipment. With no air and no atmospheric pressure, the human body can’t survive for long without some form of protection, but what happens exactly ? Do your eyes explode, does your body blow up like a balloon, and does your blood start boiling? These are some of the common ideas of what occurs in the vacuum of space, but is that what actually occurs?

The truth is less dramatic, but even more fascinating. Although you might have only seen this in movies, accidents have happened that have been invaluable to us in terms of the knowledge acquired. There have also been many tests done in controlled environments of zero pressure to simulate the conditions that one might face in space.
Chronologically, the events would occur in the following order…
Recommended Video for you:
Asphyxiation or lack of oxygen in bloodstream is the first obstacle to overcome. You wouldn’t lose consciousness straightaway, because your body would use every last bit of oxygen in your circulatory system to keep your brain functioning. This process ultimately takes about two minutes, after which the oxygen concentration drops to a critical level, making it impossible to survive without some kind of permanent injury.

You might think that a possible solution to this problem is to take in as much air as possible before being thrown out into the void, but that might be the biggest mistake you could make in this scenario. You’ll see why in a bit…
Also Read: What Would Happen If We Lost Oxygen For 5 Seconds?
Depressurization is another not so obvious, but very fatal problem. Your lungs accustom themselves to the ambient pressure over time, but a sudden pressure difference outside and inside your body is life-threatening. Comparing the high-pressure environment inside the spaceship/suit to the zero-pressure environment of space, it’s safe to say that the high-pressure gradient would cause the gas inside your lungs to expand rapidly, causing your lungs to pop almost immediately, making the most ominous sound that you’ll never hear.

What we learn from this is to follow one very important piece of advice; if you ever find yourself in such a scenario (fingers crossed you don’t…), the first thing to do when being expelled into the vacuum of space is exhale.
Also Read: Why Don’t We Get Crushed By Atmospheric Pressure?
Swelling of the skin tissue due to the loss of atmospheric pressure is another experience that you can check off your bucket list if you ever expose yourself to open space. Unfortunately, you can’t really prepare yourself for it or do anything to prevent it. After about 10 seconds of exposure, the water in your tissues will start to vaporize due to the absence of pressure holding the molecules of water close together.

You won’t “explode” though, because the human skin is quite strong and if you are returned to a normal atmospheric condition in a reasonable amount of time, you can still live out the rest of your life as a balloon. Shortly before losing consciousness due to asphyxiation, you would experience the bubbling of water on your tongue; fortunately, all the blood inside your body would be protected from vaporization by the heat provided by the circulatory system.
Also Read: What Would Happen If You Popped A Balloon In Space?
Freezing is perhaps the most obvious outcome of being stranded at almost absolute zero temperatures, but since there is no medium of heat transfer in space, you will only lose heat through radiation, which is a very slow process. Good for you? Maybe… You can’t really call it a good thing, since you would be long dead from asphyxiation by the time you would have to start worrying about frostbite.
It seems like no matter what you do, you can’t catch a single break. Outer space is brutal!

Also Read: What Is The Temperature In Outer Space?
Radiation from the sun and other cosmic bodies would also make you miss our precious ozone layer, in those few moments before you pass out. Such huge amounts of radiation would be fatal, or at least result in a truly nasty sunburn.
The fact is, unless you’re brought back into a pressurized and protected space, your body will be floating in space for a very long time. Imagine those vacuum-sealed chicken legs that you find at your supermarket. That’s close enough to what you have to look forward to becoming if you ever find yourself drifting in the void. No oxygen and no decomposition. You would have the same look on your face forever – gasping for air and waiting for the asphyxiation to set in.
In other words, don’t ever try to skinny dip in this particular ocean of “stars”. This guy knows what I’m talking about.

- Effect of spaceflight on the human body - Wikipedia. Wikipedia
- What Happens to the Human Body in Space? - NASA. The National Aeronautics and Space Administration
- What Happens to the Human Body in Space? | Science. Smithsonian
- What would happen if a human were exposed to space without .... Cornell University
- The human body in space: Distinguishing fact from fiction. Harvard University
- Survival in Space Unprotected Is Possible--Briefly. Scientific American
Harsh Gupta graduated from IIT Bombay, India with a Bachelors degree in Chemical Engineering. His pedantic and ‘know-it-all’ nature made it impossible for him not to spread knowledge about (hopefully) interesting topics. He likes movies, music and does not shy away from talking and writing about that too.

How Long Can You Survive On Different Celestial Bodies Without A Space Suit?

How Long Can An Astronaut Survive In Their Spacesuit In Open Space?

If You Die In Space, Does Your Body Decompose?

Can Babies Be Born In Space?

Can Spaceships Actually Explode Like They Do In Movies?

Switching Off Earth’s Gravity For 5 Seconds: What Would Happen?

Why Is Space Cold If There Are So Many Stars?

How Long Would You Survive On Each Planet?


How Did We Discover There's NO Oxygen in Space WITHOUT Modern Tech?

Can You Survive Jumping Out Of A Plane without A Parachute?

How Long Can An Astronaut SURVIVE In Their Spacesuit In Open Space?

What Happens to a Human at the Bottom of the Ocean?
The Science History Institute will close at 4pm on Friday, September 27 and reopen at 5pm for Ring the Bell, It’s Lunchtime! An Opening Celebration

Distillations magazine
More than 350 years ago the very first air pump changed how science was done.

In the mid-17th century Robert Boyle, with the help of Robert Hooke, set about building an air pump and with it a whole system of experimental natural philosophy. Boyle’s air pump, or vacuum chamber, created a space for experimentation on air, and it became the expensive centerpiece of a new scientific organization, the Royal Society of London. New Experiments Physico-Mechanical, Touching the Spring of the Air and Its Effects (1660) described the experiments and Boyle’s attempts to understand the nature of air, its effects on animals and respiration, and the relationship between the pressure (“spring of the air”) and volume of gas in a closed system.
Two hundred years later, air pumps still played an important role in science, but that role had changed. In the 1851 Great Exhibition at the Crystal Palace in London’s Hyde Park—a world’s fair of sorts—there were nine British exhibitors of air pumps, as well as two exhibitors from France and one from Denmark. Why? Spectacle.
Nineteenth-century air pumps made their home among other scientific instruments used to entertain, including Leyden jars, hydroelectric machines, galvanic piles, and magic lanterns. Large crowds would gather for magic-lantern shows and thrilling electrical displays meant to showcase the power of nature. Scientists of that time were often masterful performers. Humphry Davy and Michael Faraday, among others, cultivated their abilities as entertainers, enchanting crowds while increasing their own scientific fame.

The air pump in the Museum at CHF was made for this sort of public exhibition—as the name of its manufacturer, Franklin Educational Company, might suggest. The manufacturer even offered accessories, like a bell to be rung in the evacuated chamber to show that without air, it would make no sound. Such knowledge was not being tested anew; the results were predictable and were displayed for an audience’s entertainment.
There might seem a great distance between Boyle’s experimental use of the air pump in front of a small audience of like-minded natural philosophers in Oxford and the widespread public, educational uses of air pumps around the world, but they are of the same tradition. Boyle and his air pump helped change early modern science by insisting on the importance of using the senses and of witnessing nature. And for those who were not there to see for themselves, Boyle’s writing reflected painstakingly (and sometimes painfully) detailed accounts of these experiments. By the 19th century talented performers used more affordable models of the air pump to achieve the same end, bringing the sensory experiences of science to broader audiences. While nature may abhor vacuums, 19th-century audiences loved them.
Carin Berkowitz was director of the Institute’s Beckman Center for the History of Chemistry.
More from our magazine

We’re Going to Work Miracles
The failed promises of Project Plowshare.

A Tragedy with No End
Why does Garrett Hardin’s pessimistic fable haunt our collective imagination?

That Time Demons Possessed the Telegraph
Solar storms from long ago have become the delight of some scientists—and the dread of others.
Copy the above HTML to republish this content. We have formatted the material to follow our guidelines, which include our credit requirements. Please review our full list of guidelines for more information. By republishing this content, you agree to our republication requirements.
Lucy McRae experiments with negative pressure to "prep humans to go to space"
Dezeen and MINI Frontiers: artist Lucy McRae shows us the experiments she's been doing with emergency blankets and vacuum cleaners in our second behind-the-scenes preview of next week's Dezeen and MINI Frontiers exhibition .
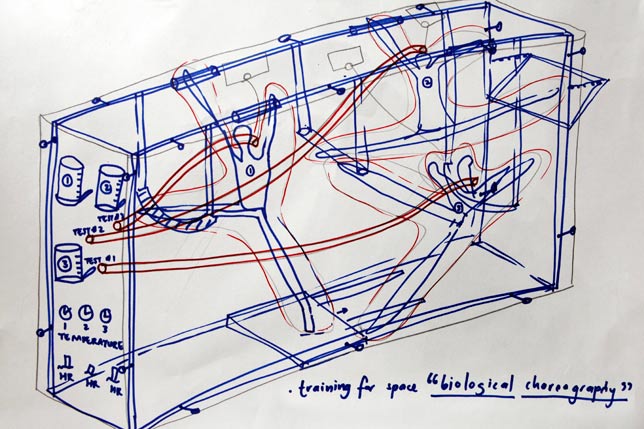
McRae 's piece for the exhibition is a speculative vacuum chamber designed to prepare the human body for long-distance space travel.
Interplanetary travel will become common, McRae believes, but first we need to develop ways of helping the body withstand the rigours of zero gravity during lengthy journeys through space.
"NASA has been developing these lower body negative pressure devices used to increase bloodflow [and] reduce hypertension," she explains. "There are all these different body improvements happening through pressure around the body. So I'm letting that medical or science story give me the leads for how to develop my concept."

McRae has been working out the best way to create her installation by vacuum-packing herself in different plastic membranes, including the emergency blankets that are often used by marathon runners after a race.
Dezeen Book of Interviews: our new book, featuring conversations with 45 leading figures in architecture and design, is on sale now
"The material kind of drips off the body like a metal, which is weirdly beautiful," McRae says. "I like the fact that you're kind of getting into a surface or a wall, so I'd like to play around with creating a huge big membrane that people can get inside of."

McRae also wants to enhance people's senses while they are in the chamber and has been experimenting with a foetal Doppler, used to detect a baby's heartbeat when it's in the womb.
"I want to create this feeling of pressure around the skin but then also amplify the senses of the people inside," she says. "I have no idea how I'm going to do that yet. But this [Doppler] is my new toy."

The Dezeen and MINI Frontiers exhibition will take place at designjunction during London Design Festival from 17 to 20 September 2014.
The music featured in the movie is a track called Contemphasic by Bankcee. You can listen to more original music on Dezeen Music Project .
Dezeen and MINI Frontiers is a year-long collaboration with MINI exploring how design and technology are coming together to shape the future.
- Technology videos
- London Design Festival
- Videos by Dezeen
- Design videos
- Installations
- Dezeen and MINI Frontiers
Subscribe to our newsletters
A quarterly newsletter rounding up a selection of recently launched products by designers and studios, published on Dezeen Showroom.
Our most popular newsletter, formerly known as Dezeen Weekly, is sent every Tuesday and features a selection of the best reader comments and most talked-about stories. Plus occasional updates on Dezeen’s services and breaking news.
Sent every Thursday and containing a selection of the most important news highlights. Plus occasional updates on Dezeen’s services and invitations to Dezeen events.
A daily newsletter containing the latest stories from Dezeen.
Daily updates on the latest design and architecture vacancies advertised on Dezeen Jobs. Plus occasional news.
Weekly updates on the latest design and architecture vacancies advertised on Dezeen Jobs. Plus occasional news.
News about our Dezeen Awards programme, including entry deadlines and announcements. Plus occasional updates.
News from Dezeen Events Guide, a listings guide covering the leading design-related events taking place around the world. Plus occasional updates and invitations to Dezeen events.
News about our Dezeen Awards China programme, including entry deadlines and announcements. Plus occasional updates.
We will only use your email address to send you the newsletters you have requested. We will never give your details to anyone else without your consent. You can unsubscribe at any time by clicking on the unsubscribe link at the bottom of every email, or by emailing us at [email protected] .
For more details, please see our privacy notice .
You will shortly receive a welcome email so please check your inbox.
You can unsubscribe at any time by clicking the link at the bottom of every newsletter.
An editorially independent publication supported by the Simons Foundation.
Get the latest news delivered to your inbox.
Type search term(s) and press enter
- Comment Comments
- Save Article Read Later Read Later
Physicists Use Quantum Mechanics to Pull Energy out of Nothing
February 22, 2023

The new quantum protocol effectively borrows energy from a distant location and thus violates no sacred physical principles.
Kristina Armitage/ Quanta Magazine
Introduction
For their latest magic trick, physicists have done the quantum equivalent of conjuring energy out of thin air. It’s a feat that seems to fly in the face of physical law and common sense.
“You can’t extract energy directly from the vacuum because there’s nothing there to give,” said William Unruh , a theoretical physicist at the University of British Columbia, describing the standard way of thinking.
But 15 years ago, Masahiro Hotta , a theoretical physicist at Tohoku University in Japan, proposed that perhaps the vacuum could, in fact, be coaxed into giving something up.
At first, many researchers ignored this work, suspicious that pulling energy from the vacuum was implausible, at best. Those who took a closer look, however, realized that Hotta was suggesting a subtly different quantum stunt. The energy wasn’t free; it had to be unlocked using knowledge purchased with energy in a far-off location. From this perspective, Hotta’s procedure looked less like creation and more like teleportation of energy from one place to another — a strange but less offensive idea.
“That was a real surprise,” said Unruh, who has collaborated with Hotta but has not been involved in energy teleportation research. “It’s a really neat result that he discovered.”
Now in the past year, researchers have teleported energy across microscopic distances in two separate quantum devices, vindicating Hotta’s theory. The research leaves little room for doubt that energy teleportation is a genuine quantum phenomenon.
“This really does test it,” said Seth Lloyd , a quantum physicist at the Massachusetts Institute of Technology who was not involved in the research. “You are actually teleporting. You are extracting energy.”
Quantum Credit
The first skeptic of quantum energy teleportation was Hotta himself.
In 2008, he was searching for a way of measuring the strength of a peculiar quantum mechanical link known as entanglement , where two or more objects share a unified quantum state that makes them behave in related ways even when separated by vast distances. A defining feature of entanglement is that you must create it in one fell swoop. You can’t engineer the related behavior by messing around with one object and the other independently, even if you call up a friend at the other location and tell them what you did.
Masahiro Hotta proposed the quantum energy teleportation protocol in 2008.
Courtesy of Masahiro Hotta
While studying black holes, Hotta came to suspect that an exotic occurrence in quantum theory — negative energy — could be the key to measuring entanglement. Black holes shrink by emitting radiation entangled with their interiors, a process that can also be viewed as the black hole swallowing dollops of negative energy. Hotta noted that negative energy and entanglement appeared to be intimately related. To strengthen his case, he set out to prove that negative energy — like entanglement — could not be created through independent actions at distinct locations.
Hotta found, to his surprise, that a simple sequence of events could, in fact, induce the quantum vacuum to go negative — giving up energy it didn’t appear to have. “First I thought I was wrong,” he said, “so I calculated again, and I checked my logic. But I could not find any flaw.”
The trouble arises from the bizarre nature of the quantum vacuum, which is a peculiar type of nothing that comes dangerously close to resembling a something. The uncertainty principle forbids any quantum system from settling down into a perfectly quiet state of exactly zero energy. As a result, even the vacuum must always crackle with fluctuations in the quantum fields that fill it. These never-ending fluctuations imbue every field with some minimum amount of energy, known as the zero-point energy. Physicists say that a system with this minimal energy is in the ground state. A system in its ground state is a bit like a car parked on the streets of Denver. Even though it’s well above sea level, it can’t go any lower.
And yet, Hotta seemed to have found an underground garage. To unlock the gate, he realized, he had only to exploit an intrinsic entanglement in the crackling of the quantum field.
The incessant vacuum fluctuations cannot be used to power a perpetual motion machine, say, because the fluctuations at a given location are completely random. If you imagine hooking up a fanciful quantum battery to the vacuum, half the fluctuations would charge the device while the other half would drain it.
But quantum fields are entangled — the fluctuations in one spot tend to match fluctuations in another spot. In 2008, Hotta published a paper outlining how two physicists, Alice and Bob, might exploit these correlations to pull energy out of the ground state surrounding Bob. The scheme goes something like this.
Bob finds himself in need of energy — he wants to charge that fanciful quantum battery — but all he has access to is empty space. Fortunately, his friend Alice has a fully equipped physics lab in a far-off location. Alice measures the field in her lab, injecting energy into it there and learning about its fluctuations. This experiment bumps the overall field out of the ground state, but as far as Bob can tell, his vacuum remains in the minimum-energy state, randomly fluctuating.
But then Alice texts Bob her findings about the vacuum around her location, essentially telling Bob when to plug in his battery. After Bob reads her message, he can use the newfound knowledge to prepare an experiment that extracts energy from the vacuum — up to the amount injected by Alice.
“That information allows Bob, if you want, to time the fluctuations,” said Eduardo Martín-Martínez , a theoretical physicist at the University of Waterloo and the Perimeter Institute who worked on one of the new experiments. (He added that the notion of timing is more metaphorical than literal, due to the abstract nature of quantum fields.)
Bob can’t extract more energy than Alice put in, so energy is conserved. And he lacks the necessary knowledge to extract the energy until Alice’s text arrives, so no effect travels faster than light. The protocol doesn’t violate any sacred physical principles.
Nevertheless, Hotta’s publication was met with crickets. Machines that exploit the zero-point energy of the vacuum are a mainstay of science fiction, and his procedure rankled physicists tired of fielding crackpot proposals for such devices. But Hotta felt certain he was onto something, and he continued to develop his idea and promote it in talks. He received further encouragement from Unruh, who had gained prominence for discovering another odd vacuum behavior .
“This kind of stuff is almost second nature to me,” Unruh said, “that you can do strange things with quantum mechanics.”
Hotta also sought a way to test it. He connected with Go Yusa, an experimentalist specializing in condensed matter at Tohoku University. They proposed an experiment in a semiconductor system with an entangled ground state analogous to that of the electromagnetic field.
But their research has been repeatedly delayed by a different kind of fluctuation. Soon after their initial experiment was funded, the March 2011 Tohoku earthquake and tsunami devastated the eastern coast of Japan — including Tohoku University. In recent years, further tremors damaged their delicate lab equipment twice. Today they are once more starting essentially from scratch.
Making the Jump
In time, Hotta’s ideas also took root in a less earthquake-prone part of the globe. At Unruh’s suggestion, Hotta gave a lecture at a 2013 conference in Banff, Canada. The talk captured the imagination of Martín-Martínez. “His mind works differently from everybody else,” Martín-Martínez said. “He’s a person that has a lot of out-of-the-box ideas that are extremely creative.”
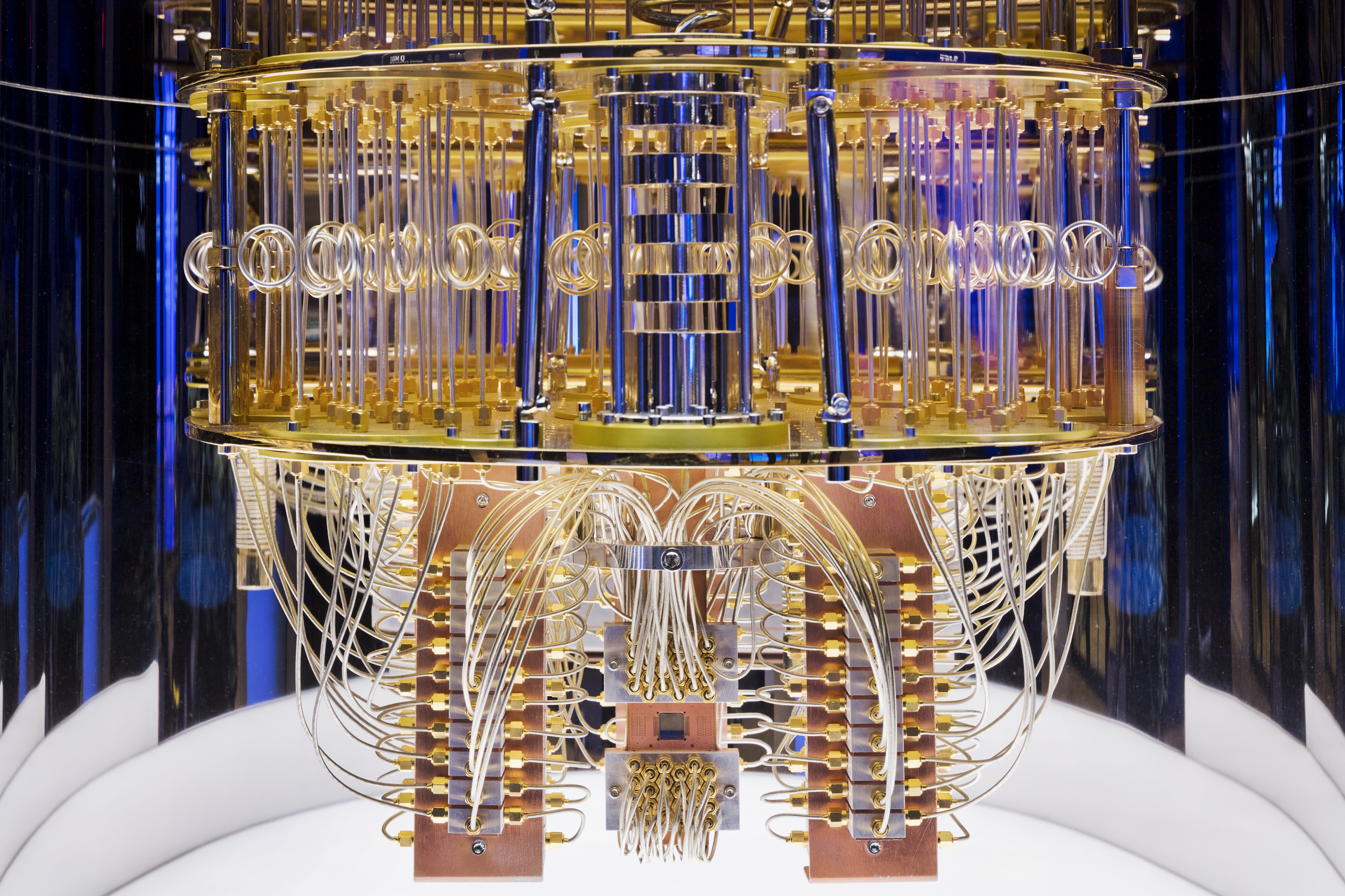
An experimental test of the teleportation protocol was run on one of IBM’s quantum computers, seen here at the Consumer Electronics Show in Las Vegas in 2020.
Martín-Martínez, who half-seriously styles himself as a “space-time engineer,” has long felt drawn to physics at the edge of science fiction. He dreams of finding physically plausible ways of creating wormholes, warp drives and time machines. Each of these exotic phenomena amounts to a bizarre shape of space-time that is permitted by the extremely accommodating equations of general relativity. But they are also forbidden by so-called energy conditions, a handful of restrictions that the renowned physicists Roger Penrose and Stephen Hawking slapped on top of general relativity to stop the theory from showing its wild side.
Chief among the Hawking-Penrose commandments is that negative energy density is forbidden. But while listening to Hotta’s presentation, Martín-Martínez realized that dipping below the ground state smelled a bit like making energy negative . The concept was catnip to a fan of Star Trek technologies, and he dove into Hotta’s work.
He soon realized that energy teleportation could help solve a problem faced by some of his colleagues in quantum information, including Raymond Laflamme , a physicist at Waterloo, and Nayeli Rodríguez-Briones , Laflamme’s student at the time. The pair had a more down-to-earth goal: to take qubits, the building blocks of quantum computers, and make them as cold as possible. Cold qubits are reliable qubits, but the group had run into a theoretical limit beyond which it seemed impossible to pull out any more heat — much as Bob confronted a vacuum from which energy extraction seemed impossible.
In his first pitch to Laflamme’s group, Martín-Martínez faced a lot of skeptical questions. But as he addressed their doubts, they became more receptive. They started studying quantum energy teleportation, and in 2017 they proposed a method for spiriting energy away from qubits to leave them colder than any other known procedure could make them. Even so, “it was all theory,” Martín-Martínez said. “There was no experiment.”
Martín-Martínez and Rodríguez-Briones, together with Laflamme and an experimentalist, Hemant Katiyar , set out to change that.
They turned to a technology known as nuclear magnetic resonance, which uses mighty magnetic fields and radio pulses to manipulate the quantum states of atoms in a large molecule. The group spent a few years planning the experiment, and then over a couple of months in the midst of the pandemic Katiyar arranged to teleport energy between two carbon atoms playing the roles of Alice and Bob.
First, a finely tuned series of radio pulses put the carbon atoms into a particular minimum-energy ground state featuring entanglement between the two atoms. The zero-point energy for the system was defined by the initial combined energy of Alice, Bob and the entanglement between them.
Next, they fired a single radio pulse at Alice and a third atom, simultaneously making a measurement at Alice’s position and transferring the information to an atomic “text message.”
Finally, another pulse aimed at both Bob and the intermediary atom simultaneously transmitted the message to Bob and made a measurement there, completing the energy chicanery.
They repeated the process many times, making many measurements at each step in a way that allowed them to reconstruct the quantum properties of the three atoms throughout the procedure. In the end, they calculated that the energy of the Bob carbon atom had decreased on average, and thus that energy had been extracted and released into the environment. This happened despite the fact that the Bob atom always started out in its ground state. From start to finish, the protocol took no more than 37 milliseconds. But for energy to have traveled from one side of the molecule to the other, it normally would have taken more than 20 times longer — approaching a full second. The energy spent by Alice allowed Bob to unlock otherwise inaccessible energy.
“It was very neat to see that with current technology it’s possible to observe the activation of energy,” said Rodríguez-Briones, who is now at the University of California, Berkeley.
They described the first demonstration of quantum energy teleportation in a preprint that they posted in March 2022; the research has since been accepted for publication in Physical Review Letters .

Nayeli Rodríguez-Briones thinks that these systems can be used to study heat, energy and entanglement in quantum systems.
Institute for Quantum Computing/University of Waterloo
The second demonstration would follow 10 months later.
A few days before Christmas, Kazuki Ikeda , a quantum computation researcher at Stony Brook University, was watching a YouTube video that mentioned wireless energy transfer. He wondered if something similar could be done quantum mechanically. He then remembered Hotta’s work — Hotta had been one of his professors when he was an undergraduate at Tohoku University — and realized he could run a quantum energy teleportation protocol on IBM’s quantum computing platform.
Over the next few days, he wrote and remotely executed just such a program. The experiments verified that the Bob qubit dropped below its ground-state energy. By January 7, he had posted his results in a preprint.
Nearly 15 years after Hotta first described energy teleportation, two simple demonstrations less than a year apart had proved it was possible.
“The experimental papers are nicely done,” Lloyd said. “I was kind of surprised that nobody did it sooner.”
Sci-Fi Dreams
And yet, Hotta is not yet completely satisfied.
He praises the experiments as an important first step. But he views them as quantum simulations, in the sense that the entangled behavior is programmed into the ground state — either through radio pulses or through quantum operations in IBM’s devices. His ambition is to harvest zero-point energy from a system whose ground state naturally features entanglement in the same way that the fundamental quantum fields that permeate the universe do.
To that end, he and Yusa are forging ahead with their original experiment. In the coming years, they hope to demonstrate quantum energy teleportation in a silicon surface featuring edge currents with an intrinsically entangled ground state — a system with behavior closer to that of the electromagnetic field.
In the meantime, each physicist has their own vision of what energy teleportation might be good for. Rodríguez-Briones suspects that in addition to helping stabilize quantum computers, it will continue to play an important role in the study of heat, energy and entanglement in quantum systems. In late January, Ikeda posted another paper that detailed how to build energy teleportation into the nascent quantum internet .
Martín-Martínez continues to chase his sci-fi dreams. He has teamed up with Erik Schnetter , an expert in general relativity simulations at the Perimeter Institute, to calculate exactly how space-time would react to particular arrangements of negative energy.
Some researchers find his quest intriguing. “That’s a laudable goal,” Lloyd said with a chuckle. “In some sense it would be scientifically irresponsible not to follow up on this. Negative energy density has very important consequences.”
Others caution that the road from negative energies to exotic shapes of space-time is winding and uncertain. “Our intuition for quantum correlations is still being developed,” Unruh said. “One constantly gets surprised by what is actually the case once one is able to do the calculation.”
Hotta, for his part, doesn’t spend too much time thinking about sculpting space-time. For now, he feels pleased that his quantum correlation calculation from 2008 has established a bona fide physical phenomenon.
“This is real physics,” he said, “not science fiction.”
Get highlights of the most important news delivered to your email inbox
Also in Physics

Can Space-Time Be Saved?

Physicists Reveal a Quantum Geometry That Exists Outside of Space and Time

If the Universe Is a Hologram, This Long-Forgotten Math Could Decode It
Comment on this article.
Quanta Magazine moderates comments to facilitate an informed, substantive, civil conversation. Abusive, profane, self-promotional, misleading, incoherent or off-topic comments will be rejected. Moderators are staffed during regular business hours (New York time) and can only accept comments written in English.
Next article
Use your social network.
Forgot your password ?
We’ll email you instructions to reset your password
Enter your new password
Human Exposure to Vacuum
Geoffrey a. landis.
The quick answers to these questions are: Clarke got it about right in 2001. You would survive about a ninety seconds, you wouldn't explode, you would remain conscious for about ten seconds.
Astronaut Bowman ejected into space without a helmet
Could You Survive?
Note that this discussion covers the effect of vacuum exposure only. The decompression event itself can have disasterous effects if the person being decompressed makes the mistake of trying to hold his or her breath. This will result in rupturing of the lungs, with almost certainly fatal results. There is a good reason that it is called "explosive" decompression.
Will You Stay Conscious?
A slightly more general interest book, Survival in Space by Richard Harding, echoes this conclusion: "At altitudes greater than 45,000 feet (13,716 m), unconsciousness develops in fifteen to twenty seconds with death following four minutes or so later." and later: "monkeys and dogs have successfully recovered from brief (up to two minutes) unprotected exposures..."
Would Your Blood Boil?
Your blood is at a higher pressure than the outside environment. A typical blood pressure might be 75/120. The "75" part of this means that between heartbeats, the blood is at a pressure of 75 Torr (equal to about 100 mbar) above the external pressure. If the external pressure drops to zero, at a blood pressure of 75 Torr the boiling point of water is 46 degrees Celsius (115 F). This is well above body temperature of 37 C (98.6 F). Blood won't boil, because the elastic pressure of the blood vessels keeps it it a pressure high enough that the body temperature is below the boiling point-- at least, until the heart stops beating (at which point you have other things to worry about!). (To be more pedantic, blood pressure varies depending on where in the body it is measured, so the above statement should be understood as a generalization. However, the effect of small pockets of localized vapor is to increase the pressure. In places where the blood pressure is lowest, the vapor pressure will rise until equilibrium is reached. The net result is the same.)
Would You Freeze?
If you were exposed to space without a spacesuit, your skin would most feel slightly cool, due to water evaporating off you skin, leading to a small amount of evaporative cooling. But you wouldn't freeze solid!
Has Anybody Ever Survived Vacuum Exposure in Real Life?
Finally, posting to sci.space, Gregory Bennett discussed an actual space incident: "Incidentally, we have had one experience with a suit puncture on the Shuttle flights. On STS-37, during one of my flight experiments, the palm restraint in one of the astronaut's gloves came loose and migrated until it punched a hole in the pressure bladder between his thumb and forefinger. It was not explosive decompression, just a little 1/8 inch hole, but it was exciting down here in the swamp because it was the first injury we've ever head from a suit incident. Amazingly, the astronaut in question didn't even know the puncture had occured; he was so hopped on adrenalin it wasn't until after he got back in that he even noticed there was a painful red mark on his hand. He figured his glove was chafing and didn't worry about it.... What happened: when the metal bar punctured the glove, the skin of the astronaut's hand partially sealed the opening. He bled into space, and at the same time his coagulating blood sealed the opening enough that the bar was retained inside the hole."
Explosive Decompression
How fast will a spaceship decompress if it gets punctured.
P = P o exp[-(A/V)t*(200m/s)]
about the author
Revised 27 June 2000 Revised 7 August. 2007 copyright 2000, 2007 by Geoffrey A. Landis
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 September 2024
11-month-olds recognize the teacher–student relationship
- Ruolan Ling 1 ,
- Reiko Matsunaka 1 &
- Kazuo Hiraki 1
Scientific Reports volume 14 , Article number: 20389 ( 2024 ) Cite this article
Metrics details
- Human behaviour
- Social neuroscience
Understanding how preverbal infants perceive and differentiate the roles of teachers and students can provide profound insights into the early stages of human learning and cognition. We thus developed a paradigm based on the task order and the interactive position to determine if infants understand teacher–student relationships. Five experiments ( N = 104), reveal that, after watching the teacher agent instruct the student agent from failure to success, 11-month-old infants looked at the teacher for a longer time when they watched a new naïve agent seek information in a new ambiguous scenario. Three additional control experiments excluded alternative interpretations of infants’ selective looking, suggesting that agents’ order-related actions influenced infants’ identification. Another control experiment demonstrated the importance of the position when the teacher and the student had interactive exchanges. These findings indicate that preverbal infants can recognize the teacher–student relationship, which is crucial to accessing effective information in early life.
Similar content being viewed by others
Children transition from simple associations to explicitly reasoned social learning strategies between age four and eight
Infants recruit logic to learn about the social world
How adults understand what young children say
Introduction.
Teaching is a common method of knowledge transmission 1 , and yet the ability of preverbal infants to recognize teacher–student interactions remains largely unexplored due to their presumed lack of language comprehension. Previous research on infants’ information acquisition has focused on their tendency to preferentially turn to informants over non-informants 2 , although instances of complete non-informants are notably scarce within preverbal infants’ daily experiences. Including a non-informant agent can overlook the transmission of general knowledge or skills between models. One of the most common scenarios associated with this issue is the teacher providing information to the student, followed by an update on the state of the student’s knowledge in the ongoing task 3 . To this effect, we aim to detect whom infants will choose to seek information from when faced with two models who can both complete the tasks. In addition, the relationship between infants and models, such as if infants would trust the more reliable models, is also well explored in the literature 4 , 5 , 6 . However, when infants are not the recipients or transmitters of information but are observing teacher–student relationships as third parties, it is pertinent to verify whether they can recognize the transmission of knowledge before exhibiting any trust-related biases.
Teaching is ubiquitous in human societies 7 , 8 . Teacher–student relationships are commonly believed to be connected with two interrelated cognitive components: (i) a perceived knowledge difference between agents 9 and (ii) one agent’s intentional activity to increase another’s knowledge or skills 10 , 11 . 11-month-olds appear to possess at least some of the cognitive abilities to comprehend these components. For component (i), by 6 to 9 months, human infants understand the meanings of many common nouns, providing a foundation for their comprehension of new knowledge in their daily lives 12 . We also know that infants use the theory of mind and social evaluation skills to understand others’ interactions 13 , 14 , being also characterized by adeptness in maintaining selective and interpretive viewpoints within diverse learning environments 15 . Clearly, infants are able to cognitively recognize individuals’ knowledge differences based on the context. For component (ii), preverbal infants can not only interpret verbal language (including turn-taking exchange sequences) to be indicative of communicating goal-relevant information between agents 16 , 17 but also make inferences, including updating a recipient’s beliefs and information states 18 , 19 , 20 . This is because they comprehend the communicative aspects of speech and recognize information-transferring conditions 21 , which are the vital components of effective communication between teachers and students 22 . Also, teaching is a kind of intentional helping behavior. Infants understand other individuals’ needs before they start helping others 23 and assign positive and negative valences to helpers and hinderers, respectively, by recognizing agents’ intentions 24 , 25 , 26 . This evidence underscores the nuanced complexity of infants’ cognitive processes, as they adeptly engage with and integrate a diverse array of stimuli, progressively building their understanding of the world around them.
A common manifestation of access to information is that infants observe adaptive behaviors and produce the same or similar behaviors in the future 27 . Thus, in the relationship between teacher and student, we cannot overlook imitation behaviors. During the imitation process, the individuals being imitated remain oblivious to others, displaying a lack of awareness or even indifference toward being imitated; but teachers always play a guiding role in facilitating student learning. Infants are able to attach social significance to imitation before they can speak 28 , 29 , and they preferentially imitate adults’ intentional actions over accidental ones 22 , which suggests that they are likely to capture the teacher’s intention in pedagogical situations. Grasping the interactive intention might likely help infants distinguish the teacher from the individuals being imitated 11 .
A sequence where students initially fail, and then succeed with the help of the teacher, following the order of “student failure” → “teacher success” (demonstration) → “student success,” forms an integral part of effective information transmission from the teacher to the student. However, we cannot yet determine whether infants, as third parties, can understand models’ interactive intentions in our teaching or imitation contexts. Given that social interaction is a key component of teaching, we try to simplify the distinction between teaching and imitation by interactive positions because infants have already distinguished between face-to-face interactive roles with others and are more engaged in face-to-face interactive positions when participating as third parties 30 , 31 . Thus, this study aims to (i) test whether infants can distinguish between teachers and students and (ii) explore how order and position influence infants to comprehend teacher–student relationships. We familiarized the infants with different task orders (Experiments 1–4) and interactive positions (Experiments 1, 5) contexts, and then compared their tendency to look at each agent when they watched a naïve agent seek help in a new ambiguous scenario. If, as a third party, infants recognize an agent who was previously acting as a teacher in a similar situation, infants will look at it in anticipation of its teaching action in uncertainty.
To explore how preverbal infants comprehend teacher–student relationships, in Experiment 1, 22 11-month-old infants were tested in a context where a teacher agent instructed the student agent to successfully learn the tasks in the order of “student failure” → “teacher success” → “student success” (Fig. 1 A). After watching how the two agents finished the tasks, we anticipated that infants looked at the teacher agent when the naïve agent did not know how to deal with the new tasks (Fig. 1 B).
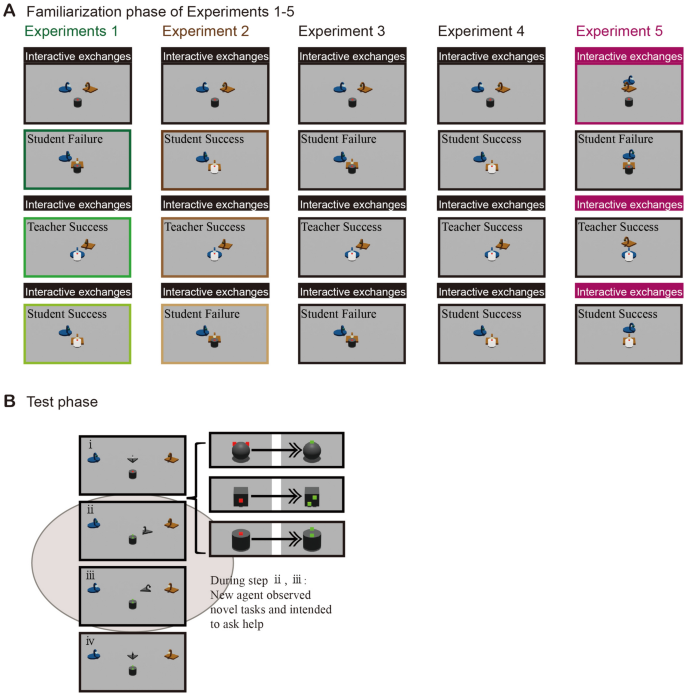
Procedures of all five experiments. ( A ) Familiarization phase. Blue represents the teacher agent, while orange represents the student agent. ( B ) Test phase. Grey represents the naïve agent.
Looking time
A paired t-test indicated that infants’ looking during the test phase (Fig. 2 A) in Experiment 1 was biased to the teacher agent ( M teacher = 0.95 s) but not to the student agent ( M student = 0.60 s) ( t (21) = 2.89, p < 0.01, Cohen’s d = 0.62) (Fig. 2 B). This result indicated that 11-month-old infants in Experiment 1 looked longer at the teacher agent when the naïve agent could not solve the new situation.
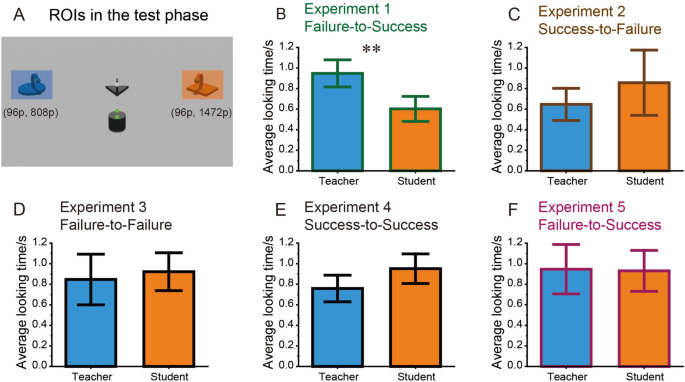
Looking time results of the five experiments. ( A ) Regions of interest (ROIs) in the test phase. ( B – F ) Looking time results in Experiments 1–5. The error bar is the standard error (SE).
We subsequently considered three alternative parsimonious interpretations of selective looking at the teacher agent before concluding that looking behaviors involved recognizing the teacher–student relationship. First, the increase in infants’ selective looking at the teacher agent in Experiment 1 might be because the teacher agent changed the student agent’s action results (from failure to success), and infants expected that the naïve agent should also follow the teacher agent’s instruction. Therefore, in Experiment 2, we tested another group of infants ( N = 20) who watched the teacher agent make the student agent fail (“student success” → “teacher success” → “student failure”). Although we only changed the order of the student agent’s action, there was no looking time difference between the two agents in Experiment 2 ( M teacher = 0.65 s, M student = 0.86 s, t (19) = − 0.89, p = 0.39) (Fig. 2 C). As such, we could exclude the alternative parsimonious interpretation that the increase in selective looks at the teacher agent was due to the teacher agent changing the student agent’s behavior.
A second possible interpretation for the increase in infant selective looks at the teacher agent could be the teacher agent’s success rate being higher than that of the student agent (teacher agent: 100%, student agent: 50%). To exclude this possibility, we conducted another two separate experiments: Experiment 3 ( N = 21) and Experiment 4 ( N = 21). In Experiment 3, infants watched the video sequence of “student failure” → “teacher success” → “student failure,” corresponding to the teacher agent having a 100% success rate and the student agent having a 0% success rate. In Experiment 4, infants watched the animation in the order of “student success” → “teacher success” → “student success,” where both the student and teacher agents had a 100% success rate. The same paired t-test in Experiment 1 was conducted in Experiments 3 and 4, and we did not find looking time differences for the student and teacher agents (Exp3: M teacher = 0.85 s, M student = 0.92 s, t (20) = − 0.36, p = 0.72; Exp4: M teacher = 0.76 s, M student = 0.95 s, t (20) = − 1.22, p = 0.24) (Fig. 2 D, E). Therefore, we could exclude the alternative parsimonious interpretation that the increase in infants’ selective looks at the teacher agent was due to the success rate difference.
In the third case, teaching would be an intentional activity to transfer effective information 10 . We conducted Experiment 5 to investigate whether infants can still detect a teacher–student relationship if we change the agents’ interactive position while keeping the task order the same. The two agents in Experiment 5 standing in line (front-to-back) instead of face-to-face (Fig. 1 A). The paired t-test showed that infants in Experiment 5 had no significant difference in looking time between the student and teacher agents ( M teacher = 0.95 s, M student = 0.93 s, t (19) = 0.07, p = 0.95) (Fig. 2 F).
Pupil dilation
To further detect infants’ comprehension of the interactive exchanges, we analyzed pupil dilation when infants watched two agents engaging in the co-dependency of exchanged triplet tones. We focused only on the first interactive exchanges at the beginning of each single video (averaging the values of the six trials), as the subsequent two interactions could be influenced by the agents’ task outcomes. The unpaired t-test showed infants had greater pupil dilation in Experiment 1 relative to Experiment 2 ( M Exp1 = 0.05 mm, M Exp2 = 0.03 mm; t’ (402.07) = 3.61, p < 0.001, Cohen’s d = 0.36), Experiment 3 ( M Exp3 = 0.02 mm; t’ (392.73) = 6.65, p < 0.001, Cohen’s d = 0.67), Experiment 4 ( M Exp4 = 0.03 mm; t’ (414) = 0.37, p = 0.001, Cohen’s d = 0.33), and Experiment 5 ( M Exp5 = − 0.00 mm; t’ (341.82) = 14.25, p < 0.001, Cohen’s d = 1.54) (Fig. 3 ). The black line in Fig. 3 indicates the specific time point of significant differences between the two groups drawn by the cluster-mass test 32 , 33 , 34 . Infants’ pupil in Experiment 1 remained larger than those of infants in Experiment 2 for intervals of 0.72 s, in Experiment 3 for intervals of 0.72 s, in Experiment 4 for intervals of 0.33 s, and in Experiment 5 for intervals of 1.67 s.

Infants’ pupil changes in the interactive exchanges of agents. The black line indicates regions of significant differences between the two groups drawn by the cluster-mass test. The error bar is the standard error (SE).
What set Experiment 2 apart from Experiment 1 was the unconventional scenario. To further explore the logical order of the teacher-student relationship as understood by 11-month-olds, we analyzed the dynamics of pupil changes and the mean y-coordinates during the familiarization phase of Experiments 1 and 2. We focused on three 3-s periods after task outcomes were displayed: student failure (SF), teacher success (TS), and student success (SS) (Fig. 4 ). These three periods were chosen because the scenes were still, and the participants watched the same scenes irrespective of Experiments 1 and 2. The only difference between the two groups was the order of the student agent’s success and failure in the Familiarization phase. Therefore, there were no potential luminosity differences between groups.
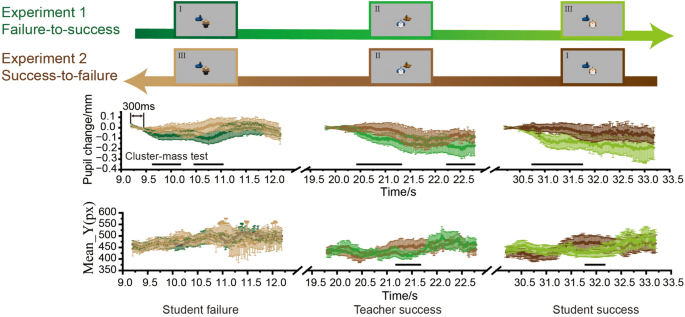
Infants’ pupil changes and mean y-coordinate in Experiments 1 and 2. The black line indicates regions of significant differences between the two groups drawn by the cluster-mass test. The error bar is the standard error (SE).
We did not analyze the pupil changes for SF, TS, and SS in Experiments 3 to 5. For Experiment 3, there was no “student success” part. Similarly, for Experiment 4, there was no “student failure” part. Experiment 5 was designed to emphasize the influence of the agent’s interactive position on infants’ cognition of the teacher–student relationship. Therefore, during the task of one agent, the other agent was standing behind in line, which is slightly different from the positions in Experiments 1 and 2. Additionally, the task order in Experiments 1 and 5 are identical, comparing pupil dilation might not effectively reflect infants’ understanding of task outcomes.
The preliminary unpaired t-test between Experiments 1 and 2 was computed on the average value (six trials) of participants’ pupil dilation and y-coordinates across the three periods. Infants’ pupillary changes were significantly greater in Experiment 2 than in Experiment 1 during the 3-s periods of SF ( M Exp1−SF = − 0.46 mm, M Exp2−SF = 0.00 mm; t’ SF (328.08) = − 15.61, p < 0.001, Cohen’s d = 1.67), TS ( M Exp1−TS = − 0.11 mm, M Exp2−TS = − 0.05 mm; t ’ TS (312.19) = − 10.47, p < 0.001, Cohen’s d = 1.10), and SS ( M Exp1−SS = − 0.12 mm, M Exp2−SS = − 0.03 mm; t ’ SS (242.42) = − 17.74, p < 0.001, Cohen’s d = 1.87). The unpaired t-test of the average value of the participants’ mean y-coordinate across the 3-s periods showed no significant difference ( M Exp1−SF = 482.65 px, M Exp2−SF = 479.87 px, t’ SF (352.92) = − 1.51, p = 0.13; M Exp1−TS = 443.78 px, M Exp2−TS = 444.04 px, t’ SS (335.10) = 0.12, p = 0.91; M Exp1−SS = 448.87 px, M Exp2−SS = 450.33 px, t’ SS (357.78) = 0.65, p = 0.52). The black line in Fig. 4 indicates the specific time point of significant differences between the two groups drawn by the cluster-mass test 32 , 33 , 34 . The pupils of the infants in Experiment 2 remained larger than those of infants in Experiment 1 over intervals of 1.15 s in the SF phase, 1.47 s in the TS phase, and 1.02 s in the SS phase; they were also inclined to look at the student agent over an interval of 0.50 s in the TS phase and at the teacher agent in an interval of 0.40 s in the SS phase.
Based on the infants’ looking time results, when the naïve agent did not know how to solve the new situation, the infants looked more at the teacher agent who helped the student agent move from failure to success (Experiment 1). Three control experiments would exclude alternative parsimonious interpretations of these increases in the selective looks toward the teacher agent: merely following the agent who changed another’s behavior (Experiment 2) and mere preferential looking at the highly successful rate agent (Experiments 3 and 4). Finally, Experiment 5 demonstrated that infants were not prone to look at the teacher agent who had the interaction in the position of front-to-back, even though the task order was the same as in Experiment 1. Evidently, the task order and interactive position influence early knowledge acquisition in the teacher-student context.
In cognitive terms, pupil dilation is a marker of memory load, attentional focus, and cognitive effort 35 , and perception of social interaction and motivation also predicts pupil dilation 36 , 37 . Teachers help students evolve from non-informants to informants in the context of new tasks, occasions, and objects, which are all rooted in the foundation of teachers’ intentional teaching. Based on infants’ pupil dilation during the agents’ interactive exchanges, the results showed the greatest pupil dilation in Experiment 1, indicating the highest level of social perception and engagement, while the weakest dilation in Experiment 5 indicated the least interactive cues. This result moderately supports that different interactive intentions between teaching and imitation could be influenced by the position of agents. Furthermore, extensive research underscores infants’ consistent prioritization of mental states 38 , 39 , 40 , 41 , 42 , 43 . In Experiment 2, when observing the task results of the teacher and student agents, infants’ larger pupil diameters indicated that they were trying to make sense of the strange “success to failure” outcomes, which were out of their expectations. Corresponding to the pupil data of the infants, the y-coordinate indicated that infants deployed a different scanning strategy during the understanding of Experiments 1 and 2. Infants inherently ascribe purpose to action 44 , 45 , 46 , 47 , 48 , 49 . Infants in Experiment 2 shifted to the agent standing behind but not the agent doing the task, indicating the agent standing behind played a particularly important role in infants’ mental processes of reasoning about these illogical tasks’ results. Thus, we posit that, at 11 months of age, infants could already integrate the agents’ non-linguistic interactive exchanges and task outcomes into integral event trajectories.
For the variations in eye movements across Experiments 1 to 5, we propose three possible explanations. The first possible explanation is that the infants might have different interpretations of interactive exchanges among the groups. Aside from Experiment 1, infants in our study did not consistently seek information from a knowledgeable agent when faced with referential uncertainty 2 , 5 , 6 , 24 . For Experiment 2, it is possible that the infants believed the teachers conveyed incorrect information to the students during interactive exchanges, leading them to perceive the teachers as untrustworthy. For Experiments 3 and 4, despite whether the student agent consistently failed or was successful, infants seemed to attribute the results to the ineffective interactive changes rather than to the unreliability of agents, which might affect infants’ evaluations of the teacher agents’ trustworthiness. Correspondingly, in Experiment 5, the interactive cues might not be perceived by infants, and they attributed the student agent’s acquisition of the information to the observation and replication of the student agent itself. Consequently, when infants employ a rational strategy to choose an effective approach to resolve their problems, the effectiveness of interactive exchanges appears to take precedence.
The second possible explanation is a teacher helping a student reach success from failure is exhibiting prosocial behavior and we cannot ignore the impact of this behavior on infants’ judgment. Currently, infants were indicated to appreciate people who help others to those who are indifferent or victimizing 4 , 18 , 50 . Although teaching is an abstract form of knowledge sharing that does not involve tangible, visual outcomes such as resource allocation, giving, or protection, our findings regarding looking time may indicate that infants in Experiment 1 recognized and appreciated the teacher’s prosocial behavior. This recognition likely led them to anticipate more prosocial actions from the teacher during the test phase. The infants’ increased looking time could therefore reflect their expectation of continued positive interactions and benevolent guidance from the teacher. In contrast, in the other groups, the teachers did not exhibit such prosocial behavior, which may have led the infants to lose interest or lower their expectations of receiving helpful or supportive actions from these teachers. As a result, the infants in these groups did not demonstrate the same level of engagement or anticipation as observed in Experiment 1, highlighting the significant impact of perceived prosocial behavior on their attention and expectations.
Thirdly, we further discuss the saliency ascribed to each agent within the task orders. The increased looking time at the student agent in all experiments except Experiment 1 suggests that the saliency of the student may be elevated for several reasons. In Experiment 2, the student’s unexpected failure on a second attempt could be interpreted as a violation of expectations by the infants. This violation increases the saliency of the student in the infants’ comprehension, leading the infants to look at the student agent during the Test phase. Similarly, in Experiment 3, the student agent failed to reproduce a successful outcome, possibly increasing their saliency and causing infants to look at the student agent. In Experiment 4, the equal success rate of both the student and teacher agents ascribed to them similar saliency. In Experiment 5, since the two agents were standing one behind the other, this unusual positioning made the student agent, who was in the front twice, more salient. However, in Experiment 1, they look at the teacher agent more because it is the most salient agent due to being informative, but the student agent lacks such competing, matching saliency, unlike in other experiments where the saliency is equal.
In sum, only in the “student failure” → “teacher success” → “student success” order and when agents stand in a face-to-face position can infants recognize the teacher–student relationship. From the results of the above five experiments, we can conclude that the human adaptation to teaching and the preparation for various social knowledge that must be learned are rooted in the infants’ minds before they are verbal.
It is also important to consider the limitations of this study. Our experimental results may be hardwired by early prosocial environmental influences. For example, the Japanese infants in our experiment were raised in a culture that tends to emphasize the development of adaptive dispositions 51 . Therefore, in future research, it is necessary to explore whether participants from different cultures are equally sensitive to the order and position in our experiment. Furthermore, children assume that those who are imitated have a higher status 52 . Although infants appeared to ask for help from an individual who helps another succeed, what types of inferences infants make about an agent’s status remain unclear. Future investigations should measure whether infants perceive different social statuses between teachers and students. If this is the case, future research should examine whether such biases influence their looking preferences. In addition, although we pilot-tested and validated the videos and measures used in the study, the effects of infants’ preference for the teacher agent were demonstrated by eye movement measured when the infants watched the screen. Future studies should examine the brain and behavioral activities of the effects of teaching using a greater range of teaching tasks and intuitive test phases, where it would be necessary to examine infants’ spontaneous behaviors without a third-party view.
This study was approved by the Ethics Committee of the University of Tokyo based on the Declaration of Helsinki (Approval No. 246-23). Parents consented to infants’ participation in the experiment and were financially compensated.
Participants
Infants were recruited based on the birth records at city hall branch offices. Parents who expressed an interest in enrolling their children in the study were contacted via email. Participants were 104 healthy full-term 11-month-old Japanese infants (48 girls; M = 333.34 days, range 296–363 days, SD = 17.52 days). An additional 17 infants were excluded from the analysis because of fussiness ( n = 3), crying ( n = 5), machine failure ( n = 2), or insufficient sampling data ( n = 7). Specifically, for Experiment 1, we recruited 27 infants and excluded 5; for Experiment 2, we recruited 24 infants and excluded 4; for Experiment 3, we recruited 24 infants and excluded 3; for Experiment 4, we recruited 23 infants and excluded 2; and for Experiment 5, we recruited 23 infants and excluded 3. Infants were randomly assigned to all experiments.
Each infant sat on their caregiver’s lap approximately 60–70 cm from a 23.8-inch monitor (EIZO FlexScan EV2451, resolution: 1920 × 1080 pixels) on which the video stimuli were presented using Tobii Pro Lab software (Tobii Technology, Stockholm, Sweden). A Tobii Pro Spectrum Eye Tracker (Tobii Technology, Stockholm, Sweden) recorded participants’ gaze movement with a 300 Hz sampling rate. The experiment was conducted in a sound-attenuated room with dimmed lights; the lighting was consistent for all participants.
Stimuli and procedure
We created video stimuli (25 fps, 1920 × 1080 pixels) using Blender 3.1 software (Blender Institute, Amsterdam, Netherlands). Each video comprised familiarization and test phases. There were three video patterns for the familiarization phase by changing the shape of the light (cylinder, sphere, or cube). Each video was presented twice in a pseudo-random order (AABBCC, AACCBB, BBAACC, BBCCAA, CCAABB, and CCBBAA) to each participant. Throughout the experimental process, our agents were non-human entities, and to avoid any individual differences induced by specific words, we used nonverbal cues instead of human language. Parents were instructed to keep the infants seated without disturbing them. After conducting a nine-point calibration procedure, we recorded infants’ eye movements.
Familiarization phase
In Experiment 1, two non-human agents approached each other and two rounds of face-to-face interactive exchanges occurred with co-dependency of exchanged triplet tones 53 (e.g., B–A–D represents the piano notes Ti–La–Re). Subsequently, the student agent (orange agent in Fig. 1 A) attempted to turn on the light but failed. The two agents then had one round of interactive exchanges (e.g., B–A–D, B–A–G) before and after the teacher agent (blue agent in Fig. 1 A) demonstrated how to turn on the light by touching the switches in a novel manner. Next, the student agent tried again and succeeded. When agents turned to speak/started engaging in the task, the ball on their top lit up to indicate that the agent was speaking/engaging in the task. The familiarization phase lasted 35 s (Supplementary Movie S1 ). In Experiment 2, the order of the student agent’s actions was changed. The student agent succeeded in the first attempt, and then failed in the second (Supplementary Movie S2 ). In Experiments 3 and 4, the student agent either consistently failed or succeeded (Supplementary Movies S3 and S4 ). In Experiment 5, we changed the position of the two agents from face-to-face to front-to-back when they did the interactive exchanges (Supplementary Movie S5 ; Fig. 1 A).
We counterbalanced the color (blue and orange) and position (left and right) of the agents as well as the order of triplet tones (“B–A–D, B–A–G,” “C–D–E, C–E–D,” “E–F–A, E–F–C,” “G–A–F, G–F–A,” 600 ms long for each triplet) in the turn-taking interactions.
Infants in all groups watched the same videos in the test phase. After the familiarization phase, there was a 500 ms black screen. Then, the student, teacher, and new naïve agents appeared. A novel object (changed from the previous one) was placed in front of the naïve agent (Fig. 1 B), and the naïve agent observed the new object but did not know how to switch on the light. Then, the naïve agent looked at and approached the other two agents sequentially and made a “hmmm” sound to express wanting help but not knowing which agent would be best to ask. During this hesitation period, the other two agents simultaneously lit up and emitted tones (similar to the triplets in the familiarization phase) to attract infants’ attention. After hesitation, the naïve agent stood still in the middle of the screen for 5 s (Fig. 1 B). The test phase lasted approximately 25 s.
The position of the naïve agent’s first observation of the light (left, right), the side of the naïve agent’s first look, and the first approach (student or teacher agents) were counterbalanced in the test phase. In every trial, we presented the familiarization phase first, and then presented the test phase. Each infant viewed these videos for a total of six trials.
Data analysis
Infants’ anticipatory looking time from the appearance of the new situation to the end of the videos was measured (from 3.8 to 25 s in the test phase, totaling 21.2 s). Infants’ original eye movement data were calculated using Tobii Pro Lab (Version 1.194). Fixation-filtered eye-tracking data (Tobii Fixation Filter with default settings) were exported for further analysis in MATLAB (Version R2021b; The MathWorks, Natick, MA).
We defined two rectangular regions of interests (ROIs) around the student and teacher agents (Fig. 2 A). A test trial was considered valid if there was at least one fixation in any ROI. Data from participants with less than 50% of detected gaze data or with less than three out of six valid trials were excluded from the final analysis. The average number of valid trials was 5.73 in Experiment 1, 5.25 in Experiment 2, 5.71 in Experiment 3, 5.48 in Experiment 4, and 5.75 in Experiment 5.
Participants’ looking time was calculated as the sum of the duration of all fixations that fell into the ROIs in a single video. The looking time was averaged over six trials in each group.
The baseline of pupil diameter was computed by averaging the first 300 ms of each period, which was considered sufficient for the physiological response of the pupil to adapt to lighting variations 33 . We then subtracted the baseline pupil diameter from the raw pupil diameter to calculate the relative value of the target periods and averaged the values over the six trials for each individual to reflect the actual pupil changes of every participant. Within each period, the y-coordinate of infants’ eyes revealed whom they were looking at during a given moment, which was considered an auxiliary reference for the pupil change results to explain the infants’ cognitive processing of the teacher–student relationship. Finally, a cluster-mass test 54 was used to locate the specific time points of cognitive distinctions following previous studies 32 , 33 , 34 . The threshold of the t-values was 1.6 32 .
Data availability
The data can be available upon request from the corresponding author.
Caro, T. M. & Hauser, M. D. Is there teaching in nonhuman animals?. Q. Rev. Biol. 67 , 151–174 (1992).
Article CAS PubMed Google Scholar
Bazhydai, M., Westermann, G. & Parise, E. I don’t know but I know who to ask: 12-month‐olds actively seek information from knowledgeable adults. Dev. Sci. 23 , e12938 (2020).
Article PubMed Google Scholar
Corbett, A. T. & Anderson, J. R. Knowledge tracing: Modeling the acquisition of procedural knowledge. User Model. User-Adapt. Interact. 4 , 253–278 (1994).
Article Google Scholar
Zmyj, N., Buttelmann, D., Carpenter, M. & Daum, M. M. The reliability of a model influences 14-month-olds’ imitation. J. Exp. Child. Psychol. 106 , 208–220 (2010).
Brosseau-Liard, P. E. & Poulin‐Dubois, D. Sensitivity to confidence cues increases during the second year of life. Infancy 19 , 461–475 (2014).
Crivello, C., Phillips, S. & Poulin-Dubois, D. Selective social learning in infancy: Looking for mechanisms. Dev. Sci. 21 , e12592 (2018).
Thornton, A. & McAuliffe, K. Teaching in wild meerkats. Science 313 , 227–229 (2006).
Article ADS CAS PubMed Google Scholar
Kline, M. A. How to learn about teaching: An evolutionary framework for the study of teaching behavior in humans and other animals. Behav. Brain Sci. 38 , e31 (2015).
Knutsen, J., Mandell, D. S. & Frye, D. Children with autism are impaired in the understanding of teaching. Dev. Sci. 20 , e12368 (2017).
Kruger, A., Tomasello, M., Olson, D. & Torrance, N. Handbook of Education and Human Development: New Models of Learning, Teaching, and Schooling (1996).
Ziv, M., Solomon, A. & Frye, D. Young children’s recognition of the intentionality of teaching. Child. Dev. 79 , 1237–1256 (2008).
Bergelson, E. & Swingley, D. At 6–9 months, human infants know the meanings of many common nouns. Proc. Natl. Acad. Sci. USA 109 , 3253–3258 (2012).
Article ADS CAS PubMed PubMed Central Google Scholar
Kiley Hamlin, J., Ullman, T., Tenenbaum, J., Goodman, N. & Baker, C. The mentalistic basis of core social cognition: Experiments in preverbal infants and a computational model. Dev. Sci. 16 , 209–226 (2013).
Article CAS PubMed PubMed Central Google Scholar
Meristo, M. & Surian, L. Do infants detect indirect reciprocity?. Cognition 129 , 102–113 (2013).
Bransford, J. D., Brown, A. L. & Cocking, R. R. How People Learn: Brain, Mind, Experience, and School: Expanded Edition (National Academies, 2000).
Google Scholar
Tauzin, T. & Gergely, G. Communicative mind-reading in preverbal infants. Sci. Rep. 8 , 9534 (2018).
Article ADS PubMed PubMed Central Google Scholar
Tauzin, T. & Gergely, G. Variability of signal sequences in turn-taking exchanges induces agency attribution in 10.5-mo-olds. Proc. Natl. Acad. Sci. USA 116 , 15441–15446 (2019).
Onishi, K. H. & Baillargeon, R. Do 15-month-old infants understand false beliefs?. Science 308 , 255–258 (2005).
Southgate, V., Chevallier, C. & Csibra, G. Seventeen-month‐olds appeal to false beliefs to interpret others’ referential communication. Dev. Sci. 13 , 907–912 (2010).
Song, H., Onishi, K. H., Baillargeon, R. & Fisher, C. Can an agent’s false belief be corrected by an appropriate communication? Psychological reasoning in 18-month-old infants. Cognition 109 , 295–315 (2008).
Article PubMed PubMed Central Google Scholar
Martin, A., Onishi, K. H. & Vouloumanos, A. Understanding the abstract role of speech in communication at 12 months. Cognition 123 , 50–60 (2012).
Carpenter, M., Akhtar, N. & Tomasello, M. Fourteen through 18-month-old infants differentially imitate intentional and accidental actions. Infant Behav. Dev. 21 , 315–330 (1998).
Köster, M., Ohmer, X., Nguyen, T. D. & Kärtner, J. Infants understand others’ needs. Psychol. Sci. 27 , 542–548 (2016).
Kuhlmeier, V., Wynn, K. & Bloom, P. Attribution of dispositional states by 12-month-olds. Psychol. Sci. 14 , 402–408 (2003).
Hamlin, J. K., Wynn, K. & Bloom, P. Social evaluation by preverbal infants. Nature 450 , 557–559 (2007).
Salvadori, E. et al. Probing the strength of infants’ preference for helpers over hinderers: Two replication attempts of Hamlin and Wynn (2011). PLoS One 10 , e0140570 (2015).
Csibra, G. & Gergely, G. Natural pedagogy. Trends Cogn. Sci. 13 , 148–153 (2009).
Powell, L. J. & Spelke, E. S. Human infants’ understanding of social imitation: Inferences of affiliation from third party observations. Cognition 170 , 31–48 (2018).
Powell, L. J. & Spelke, E. S. Third-party preferences for imitators in preverbal infants. Open. Mind 2 , 61–71 (2018).
Gustafsson, E. et al. Do infants recognize engagement in social interactions? The case of face-to‐face conversation. Infancy 21 , 685–696 (2016).
Augusti, E. M., Melinder, A. & Gredebäck, G. Look who’s talking: Pre-verbal infants’ perception of face-to-face and back-to-back social interactions. Front. Psychol. 1 , 2076 (2010).
Hochmann, J. R. & Papeo, L. The invariance problem in infancy: A pupillometry study. Psychol. Sci. 25 , 2038–2046 (2014).
Cesana-Arlotti, N. et al. Precursors of logical reasoning in preverbal human infants. Science 359 , 1263–1266 (2018).
Hochmann, J. R. & Toro, J. M. Negative mental representations in infancy. Cognition 213 , 104599 (2021).
Kahneman, D. & Beatty, J. Pupil diameter and load on memory. Science 154 , 1583–1585 (1966).
Cheng, Y., Liu, W., Yuan, X. & Jiang, Y. The eyes have it: Perception of social interaction unfolds through pupil dilation. Sci. Rep. 11 , 1595–1598 (2021).
ADS Google Scholar
Zeng, G. et al. Social motivation predicts gaze following between 6 and 14 months. Sci. Rep. 13 , 836–860 (2023).
Legerstee, M. & Markova, G. Intentions make a difference: Infant responses to still-face and modified still-face conditions. Infant Behav. Dev. 30 , 232–250 (2007).
Marsh, H. L., Stavropoulos, J., Nienhuis, T. & Legerstee, M. Six-and 9‐month‐old infants discriminate between goals despite similar action patterns. Infancy 15 , 94–106 (2010).
Hamlin, J. K., Newman, G. E. & Wynn, K. Eight-month-old infants infer unfulfilled goals, despite ambiguous physical evidence. Infancy 14 , 579–590 (2009).
Hamlin, J. K., Hallinan, E. V. & Woodward, A. L. Do as I do: 7-Month‐old infants selectively reproduce others’ goals. Dev. Sci. 11 , 487–494 (2008).
Brandone, A. C. & Wellman, H. M. You can’t always get what you want: Infants understand failed goal-directed actions. Psychol. Sci. 20 , 85–91 (2009).
Behne, T., Carpenter, M., Call, J. & Tomasello, M. Unwilling versus unable: Infants’ understanding of intentional action. Dev. Psychol. 41 , 328–337 (2005).
Baldwin, D. A. Early referential understanding—Infants ability to recognize referential acts for what they are. Dev. Psychol. 29 , 832–843 (1993).
Leslie, A. M. ToMM, ToBy, and agency: Core architecture and domain specificity (Cambridge University Press, 1994).
Gergely, G., Nadasdy, Z., Csibra, G. & Biro, S. Taking the intentional stance at 12 months of age. Cognition 56 , 165–193 (1995).
Meltzoff, A. N. Understanding the intentions of others: Re-enactment of intended acts by 18-month-old children. Dev. Psychol. 31 , 838–850 (1995).
Woodward, A. L. Infants selectively encode the goal object of an actor’s reach. Cognition 69 , 1–34 (1998).
Gergely, G., Bekkering, H. & Kiraly, I. Rational imitation in preverbal infants. Nature 415 , 755 (2002).
Kanakogi, Y. et al. Preverbal infants affirm third-party interventions that protect victims from aggressors. Nat. Hum. Behav. 1 , 1–7 (2017).
Hess, R. D. & Azuma, H. Cultural support for schooling: Contrasts between Japan and the United States. Educ. Res. 20 , 2–9 (1991).
Over, H. & Carpenter, M. Children infer affiliative and status relations from watching others imitate. Dev. Sci. 18 , 917–925 (2015).
Tauzin, T. & Gergely, G. Co-dependency of exchanged behaviors is a cue for agency attribution in 10-month-olds. Sci. Rep. 11 , 1–7 (2021).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164 , 177–190 (2007).
Download references
Acknowledgements
This study was supported by Core Research for Evolutionary Science and Technology from the JST (Grant Number: JPMJCR18A4), the Ministry of Education, JST Moonshot R&D (Grant Number: JPMJMS2293-04), and Culture, Sports, Science and Technology (Grant Number: 211502). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We gratefully acknowledge all parents and infants for their participation.
Author information
Authors and affiliations.
Department of General System Studies, Graduate School of Arts and Sciences, The University of Tokyo, Tokyo, 153-8902, Japan
Ruolan Ling, Reiko Matsunaka & Kazuo Hiraki
You can also search for this author in PubMed Google Scholar
Contributions
R.L.: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing original draft & EditingR.M.: Conceptualization, Methodology, Supervision, Review. K.H.: Conceptualization, Methodology, Funding acquisition, Supervision, Review.
Corresponding authors
Correspondence to Ruolan Ling or Kazuo Hiraki .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Movie S1.
Supplementary Movie S2.
Supplementary Movie S3.
Supplementary Movie S4.
Supplementary Movie S5.
Supplementary Legends.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ling, R., Matsunaka, R. & Hiraki, K. 11-month-olds recognize the teacher–student relationship. Sci Rep 14 , 20389 (2024). https://doi.org/10.1038/s41598-024-70828-2
Download citation
Received : 06 February 2024
Accepted : 21 August 2024
Published : 25 September 2024
DOI : https://doi.org/10.1038/s41598-024-70828-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Stack Exchange Network
Stack Exchange network consists of 183 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Q&A for work
Connect and share knowledge within a single location that is structured and easy to search.
Have we managed to make a perfect vacuum?
Have we managed to make a device without any atom inside it on earth? I was reading about vacuum here , and I found in the examples part here that even on the best man made vacuum devices, there are still millions of "molecules per $cm^3$". So my other question here is, what are these molecules and what is the mass of them?
- experimental-physics
- 1 $\begingroup$ No. Every vacuum is measured with the scale given en.wikipedia.org/wiki/Free_space#Measurement , not being "Perfect Vacuum". $\endgroup$ – zonksoft Commented Jan 25, 2013 at 23:59
- $\begingroup$ In most UHV (ultra high vacuum) systems, including mine, the main component is by far hydrogen molecules (H2). They're light and very difficult to pump out. For that reason, many pumps in the UHV range focus solely on pumping hydrogen (e.g., titanium sublimation or non-evaporable getters). $\endgroup$ – emarti Commented Jan 26, 2013 at 23:29
4 Answers 4
As zonk said, there is no perfect vacuum. Even the 'vacuum' of space contains a few atoms per cubic meter on average.
In the lab, the lack of a high vacuum usually results from not having a pump that can effectively extract enough of the particles inside the chamber you're trying to evacuate.
There are several different kinds of pumps used, depending on how 'good' of a vacuum you want: mechanical roughing pumps, ion pumps, turbo-molecular pumps among others. Each works either by transferring gas particles or by capturing them. Since transfer works by preferentially forcing gas molecules in a certain direction, it cannot remove ALL the particles (as in the electrons inside a wire: on average, you can force the current preferentially in the direction of the applied voltage but each individual electron will zip around in all directions). A capture pump is better at removing particles, but it is still limited by how many materials they have already captured and can saturate.
Even if we had a perfect pump, you would still need to be careful about what is inside the chamber or what the chamber is made of. Fingerprint grease, zinc (and brass), and plastics actually out-gas under high enough vacuums. This means that the particles normally trapped under atmospheric pressures can break loose and rattle around inside your vacuum if you're not careful.
Also, pumps such as mechanical pumps use lubricating oil to function (though some fancier ones such as the ion pumps do not) and that can get into the chamber as well and increase the number of particles.
That's probably more than you wanted to know, but there you have it.
I do not think the tag 'particle physics' is accurate. Particle physics generally refers to subatomic physics and encompasses things like quarks, QED, and the Higgs boson. Perhaps 'experimental physics' would be better?
(Adding another answer as my response to Hurricane's question is too long for comments)
Glad it helped. Richard Terrett is correct, (charged) anti-matter is confined in a magnetic trap in as high of a vacuum as we can get. Uncharged anti-matter must be contained using laser traps ('optical tweezers' is something to look into if you're curious).
There will still be some particles in the antimatter container but, contrary to the DaVinci Code and popular perception, when one particle of anti-hydrogen meets a particle of hydrogen, the energy released (according to E=mc^2, which I believe is correct) is 3*10^-10 Joules or 0.3 nanojoules.
To give this a sense of scale, Wikipedia says a nanojoule (10^-9 Joules) is equal to 'about 1/160 of the kinetic energy of a flying mosquito'. So an antihydrogen annihilating with a stray hydrogen in the vessel would be have less of an effect than a mosquito bumping the outside of the container, energetically speaking.
Now, we can also determine how many particles would be in a container. Let's take the highest vacuum rating; ultra-high vacuum, which is classified as having a pressure under 10^-7 pascal, with 10^-10 pascal (10^-12 torr) being the golden standard among people who care about excellent vacuum vessels. A lot of UHV systems operate at very low temperatures, but let us take the temperature of the inside of the vessel to be room-temperature, T=293 Kelvin. We can use the ideal gas equation if we know the volume of the container, PV=nRT. Plugging in the pressure, room temperature, and assuming a container size of a rubik's cube [V=(5.5*10^-2 meters)^3=1.66*10^-4 meters^3], we arrive at a number of particles of n=6.83*10^-18 moles or 4.113*10^6 molecules. To put this in perspective, at standard pressure and temperature in the same size vessel (STP, 101325 pascal, 293K), we would have 4.155×10^21 molecules. So UHV is a pretty damn good vacuum.
- $\begingroup$ Great answer, tag edited too. Now one last question since you seem to know much about this. I have asked earlier about the mass of these particles still in there. Wikipedia mentioned molecules but I don't know molecules of what. Anyway to clarify this, I asked this question to know if there is 100% safe place to store antimatter when we somehow get to know how. So now the important thing is about the mass of these "molecules" or particles, have you got any idea about how many are left in these different vacuum methods and what the mass of them is ? $\endgroup$ – Hurricane Commented Jan 26, 2013 at 8:58
- 1 $\begingroup$ @Hurricane - Antimatter can be stored in a Penning trap . $\endgroup$ – Richard Terrett Commented Jan 26, 2013 at 9:11
- 1 $\begingroup$ @RichardTerrett, Wikipedia says it is used to store charged particles like antiprotons, so does this also apply for complete atoms like antihydrogen ones since atoms are not charged ? $\endgroup$ – Hurricane Commented Jan 26, 2013 at 23:05
- $\begingroup$ @Hurricane You should ask that as a new question (assuming it doesn't already exist on this site). $\endgroup$ – user10851 Commented Jan 26, 2013 at 23:14
The current record vacuum, made at CERN, is around 1000atoms/cubic cm
For comparison intersteller space (well away from the solar system) is around 1 atom/cc and deep intergalactic, really empty, space is around 0.001 atom/cc
The bits of space we can reach, in Earth orbit, are only about the sort of vacuum you can get in an ordinary lab vac pump.
- $\begingroup$ in Earth orbit Could you please say whether that would be low-earth orbit (LEO) or geostationary orbit (GEO), thanks. $\endgroup$ – Eugene Seidel Commented Jan 26, 2013 at 21:19
- $\begingroup$ Upto about 600km (LEO) it's the atmosphere reducing in density with height. Above that (MEO and GSO) it's mostly ions from the sun - don't remember the figures $\endgroup$ – Martin Beckett Commented Jan 26, 2013 at 21:29
I'd like to add another dimension to this discussion. Even if we were to hypothetically evacuate all atoms from a certain region of space, quantum field theory tells us that there would still be something there, namely the "zero point" energy corresponding to the ground state of fields. So in this sense, at least as far as I am aware, our current model of fundamental physics renders the existence of a region of space with "nothing" there an impossibility, even in principle. http://en.wikipedia.org/wiki/Zero-point_energy
- 4 $\begingroup$ Plus a bath of photons from the walls. $\endgroup$ – dmckee --- ex-moderator kitten Commented Jan 26, 2013 at 3:59
- 2 $\begingroup$ -1: The "zero-point energy" is referring to virtual particles, not real particles. $\endgroup$ – Chris Gerig Commented Jan 26, 2013 at 4:36
- 1 $\begingroup$ @ChrisGerig: true, but the Casimir effect shows there is something there so I think your downvote is unkind. $\endgroup$ – John Rennie Commented Jan 26, 2013 at 10:11
- 2 $\begingroup$ @JohnRennie: First sentence of question: "a device with no ANY atom inside it" ... atom... not fake particle. $\endgroup$ – Chris Gerig Commented Jan 26, 2013 at 18:15
- 2 $\begingroup$ @ChrisGerig I think you make a good point that my answer isn't immediately relevant to the body of the question, but that's why I added the phrase "add another dimension to this discussion;" I simply thought certain people might find this, admittedly somewhat tangential, answer interesting given the broader scope of the question title. Oh btw I was a physics/math undergrad at Cal; I'm not surprised you left physics for math. Cheers. $\endgroup$ – joshphysics Commented Jan 26, 2013 at 18:25
It is really really really difficult to get a vacuum of 1E-10 Pa at room temperature when surrounded by a 1E+5 Pa atmosphere (sea level on Earth). The answer referring to Penning Traps needs to be understood in the context of the life-time of the antiprotons being on the order of seconds or less. So, unless your story has a method of creating and isolating a whole lot of antimatter (so that the losses due to leakage are negligible), you're not going to be storing it for very long. OTOH, it isn't obvious to me that if you made your equipment in outer space (or say on the Moon or an asteroid) that the problems would be nearly as difficult to solve. The easiest place to store antimatter would be in outer space (more specifically, in the void between the cosmic filaments far away from any galaxy or star). On Earth, even stainless steel acts like a "wet sponge" when we're concerned with ultrahigh vacuum. We have to bake everything to drive the volatiles (water but especially gasses like hydrogen, helium, argon, nitrogen, CO2, methane, CO, etc.) out of all of the equipment. This would be easier in outer space (or the surface of the Moon). Note that even there there will be some gas molecules (atoms) around contaminating things.
- $\begingroup$ But this suggests that you could create a vacuum in a container by putting it on a satellite and use the vacuum of space to evacuate it, close it and then bring it back to Earth. $\endgroup$ – Count Iblis Commented Jun 5, 2016 at 23:01
Not the answer you're looking for? Browse other questions tagged experimental-physics vacuum or ask your own question .
- Featured on Meta
- Preventing unauthorized automated access to the network
- User activation: Learnings and opportunities
- Join Stack Overflow’s CEO and me for the first Stack IRL Community Event in...
Hot Network Questions
- All combinations of ascending/descending digits
- How would RA Fisher (or "a Fisherian") choose sample sizes?
- How old was Abraham when Rebecca (Isaac's wife) was born?
- tan 3θ = cot(−θ)
- Expected value of a matrix = matrix of expected value?
- Thunderbird will not create profile in chosen folder on a different partition
- Is there a faster way to find the positions of specific elements in a very large list?
- What is the smallest interval between two palindromic times on a 24-hour digital clock?
- Harmonizing modes other than major and minor
- Is there a name for this Fibonacci formula that uses hyperbolic trigonometry?
- How long have people been predicting the collapse of the family?
- How do you tell someone to offer something to everyone in a room by taking it physically to everyone in the room so everyone can have it?
- Does this work for page turns in a busy violin part?
- Is there an AGPL 3.0 ONLY license?
- Do mathematicians care about the validity ("truth") of the axioms?
- What is the simplest formula for calculating the circumference of a circle?
- Why is China not mentioned in the Fallout TV series despite its significant role in the games' lore?
- How many jet fighters is Azerbaijan purchasing?
- Help. It's not compiling!
- How can understanding the historical context of a biblical text change our theological conclusions about it?
- Player sprite becomes smaller during attack animation (Java)
- How do I avoid getting depressed after receiving edits?
- How similar were the MC6800 and MOS 6502?
- Is “No Time To Die” the first Bond film to feature children?

The Oikofuge
Human exposure to vacuum: part 1.
The topic of explosive decompression generates a lot of nonsense, particularly in science fiction films and television series, but also scattered across the internet generally. We actually know quite a lot about what would happen if a human being were exposed to the vacuum of space—and it turns out not to be like the movies.
For this first part, I’m going to write a bit about basic physics and physiology, and discuss what that can tell us about the accuracy (or otherwise) of the common SF tropes we see in the movies. In the second part, I’ll move on to the evidence we have from actual vacuum exposures and explosive decompressions.

Will people explode when exposed to vacuum? Absolutely not.
Liquid pressures aren’t an issue—when decompressed, the liquids in our tissues will expand only very, very slightly before their ambient pressure drops to zero. Gas pressures are the problem. Our bodies contain various gas cavities which are at the same pressure as the surrounding atmosphere. Most spacecraft and spacesuits aren’t pressurized to one atmosphere, but we can take that as the worst-case scenario for explosive decompression. So at the moment tissue pressures fall to zero, these gas cavities will press outwards against the surround tissue with one atmosphere of pressure—that’s 100 kilopascals, which is 100,000 newtons per square metre.
But skin and soft tissue is strong. Here’s film from Arthur C. Clarke’s World of Strange Powers (1985). The scientists are reproducing a traditional “hook-hanging” rite carried out at Kataragama, Sri Lanka:
The volunteer weighs 55 kilograms, and hangs from six slim hooks. With a generous allowance of 30 square centimetres for the total suspension area, that comes out to pressures of 180,000 newtons per square metre on the soft tissues the hooks supports. That’s almost twice our worse-case limit, and the skin doesn’t even stretch very far.
So no exploding.

Will people freeze solid as soon as they are exposed to space? Absolutely not.
Vacuum is a good insulator. At cool ambient temperatures, our bodies lose heat mainly by conduction and convection, which is why air temperature and wind speed are so important to the way we dress outdoors. In the absence of air, our skin will cool by radiation—the loss of energy at infrared wavelengths emitted by our warm bodies. Depending on skin temperature and clothing, we radiate at anything from 100 to several hundred watts. So that’s how fast we’ll lose heat.
Now, we’re made mainly of water, and water has a high specific heat capacity, around 4000 J/kg/ºC—which means a kilogram of water needs to lose 4000 joules to fall in temperature by one degree Celsius. So an 80-kilogram bag of water (that’s approximately me), is going to need to lose over 300,000 joules of energy before its temperature falls by one degree. (That’s neglecting the continuing metabolic production of energy in the meantime.) If I’m radiating at a generous 500 watts, and producing no internal energy, and receiving no energy from sunlight, that’s ten minutes of vacuum exposure before my temperature falls by just one degree Celsius.
There may be some local difficulties, though. We also cool by evaporation, which becomes significant when it’s hot enough to cause sweating. Water will evaporate from any moist surfaces exposed to vacuum, and it will take energy with it as it does so, driving down the temperature of the tissue it evaporates from. So on exposure to vacuum the eyes, nasal cavity, and probably mouth and respiratory tract are going to start cooling by evaporation. How cold they get will depend on how quickly water moves out of the tissues to replace what is lost from the surface.
But no-one is going to turn to ice crystals and shatter.
BREATH-HOLDING

Should people hold their breath if about to be exposed to vacuum? Not a good idea.
With the tissues equilibrated to an ambient zero pressure, the cardiovascular system will continue to work as usual—all its pressures are relative pressures (what engineers call “gauge” pressures). A blood pressure of 120/80 is telling you that the systolic pressure is 120 millimetres of mercury (mmHg) above ambient, and the diastolic pressure 80 mmHg above ambient. That’s true at sea level with an ambient pressure of one atmosphere, at twenty metres down in the ocean with an ambient pressure of three atmospheres, on top of Everest with an ambient pressure of a third of an atmosphere, or in vacuum at zero atmospheres.
Trouble is, respiratory gas exchange works on absolute pressures. No matter what your tissue ambient pressure is, you still need to breathe a partial pressure of 160 mmHg of oxygen (21% of an atmosphere) to get a normal concentration of oxygen into your blood. This is fine when you’re breathing at one atmosphere of ambient pressure. It’s even fine on top of Everest—if you mix supplementary oxygen into a third of an atmosphere of breathing gas, you can easily get normal oxygenation while still balancing the pressure inside your lungs against the ambient pressure outside your body and in your tissues. There’s no resulting pressure gradient, and nothing gets squashed or stretched.
But in a vacuum, the pressure in your lungs (necessary for gas exchange) is not balanced by any external pressure. Holding air in your chest is going to cause pressure outwards, stretching the lungs; and inwards, compressing the heart and large blood vessels in the middle of your chest. And notice that even a standard 160 mmHg pressure of oxygen is a large pressure , exceeding the normal pressures of arterial blood. It’s enough pressure to squash your heart , which is not going to have a good effect on its ability to pump. This is why people can make themselves faint while trying too hard to blow up a balloon—the high pressure inside their chest interferes with the flow of blood through the heart. So trying to hold on to a lungful of oxygen in vacuum will make your blood pressure crash, and you’ll almost certainly pass out.
And that 160 mmHg is the smallest plausible pressure someone might find themselves trying to hold when suddenly exposed to vacuum. It’s the minimum operating pressure for spacesuits—most operate at around 240 mmHg. The Space Shuttle maintained an internal atmosphere at 530 mmHg during missions. These are pretty lethal pressures to try to hold in the lungs.
It’s not just the cardiovascular system that will suffer. The lungs themselves are not designed to support that sort of pressure differential. In the fifth edition of Diving and Subaquatic Medicine , Chapter 6, Edmonds et al. report that a person’s lungs will leak air into the surrounding tissue when subjected to a pressure gradient of 110 mmHg, even if the chest is prevented from expanding using a binder. If the chest is allowed to expand in response to the imposed pressure, the lungs start to leak at just 70 mmHg. (Admittedly, this is from a cadaver study, but it’s not the sort of test you can find volunteers for.) What’s going on here is that stretch is bad for your lungs, too—if your chest is blown up like a balloon, the lungs will burst at a lower pressure. This is bad news for anyone tempted to take a deep breath before entering vacuum—the extra stretch moves their lungs closer to the burst point. But that’s probably academic, since these experimental burst pressures are lower than the lowest spacesuit operating pressures.
So if you try to hold a lungful of air on decompression, not only will you squash your heart and cause your blood pressure to fall catastrophically, your lungs will leak—they’ll squeeze air into the lung blood vessels, sending showers of bubbles into your circulation; they’ll squeeze air into the tissues around your heart and then up into your neck; and they’ll squeeze air into the pleural cavities lining your chest, causing your lungs to deflate.
As an added extra, the air held in your middle ears will burst your eardrums.
So breath-holding on exposure to vacuum is a good way to incapacitate yourself. Is there another option?

Should people exhale on exposure to vacuum? Better … but still not great.
If breath-holding will make you lose consciousness and pop your lungs, not breath-holding seems like the only viable alternative. If you can arrange to yawn just as the pressure drops, to open your Eustachian tubes and let the air out of your middle ears, then you’ll also prevent your eardrums bursting.
Exhaling gets rid of that abnormal pressure gradient in the chest, so there’s no interference with blood pressure, and no popped lungs. However, it takes time for the lungs to empty, so if decompression is very fast, lung injury could still occur while the pressure in the lungs remains transiently higher than the pressure in the surrounding tissues. Here’s a theoretical plot from the second edition of the Bioastronautics Data Book , showing that a 250 mmHg decompression (from 350 to 100 mmHg) over 0.3 seconds will produce a brief pressure gradient across the chest wall that reaches what we know to be potentially lung-popping levels:

Having exhaled to vacuum, there’s now no oxygen at all in the lungs. From the point of view of keeping oxygen circulating in the blood, this is a disaster. Venous blood, returning from the tissues, still contains a considerable residue of oxygen. This is normally topped up by oxygen diffusing from the lungs into the blood. Even if not much oxygen is added (for instance, if you’re holding your breath), at least some oxygen goes back to the tissues. But if there’s no oxygen in the lungs, the normal diffusion gradient is reversed—oxygen leaves the venous blood and diffuses into the space inside the lungs. So very little then gets sent back to the tissues. This means that tissue oxygenation fails abruptly and catastrophically—much faster than it does with simple breath-holding.
We’ve got some experience of how quickly things go badly wrong under this sort of hypoxic insult—some of it comes from pilots depressurizing at very high altitude, and some of it comes from people who have accidentally breathed gas containing no oxygen, usually in an industrial accident.
The USN Aerospace Physiologist’s Manual makes a prediction about how long a person might remain conscious and orientated enough to carry out simple tasks, once exposed to near-vacuum. This is based on the apparent convergence of a number of graphs generated by decompressing various hapless volunteers to various altitude-equivalents during the 1960s:

Somewhere around an altitude of 65,000 ft (with an air pressure of 43 mmHg, and a partial pressure of oxygen of just 9 mmHg) the period during which volunteers remain conscious enough to perform simple tasks converges on 12 seconds .
So a Time of Useful Consciousness (TUC) of 12 seconds following exposure to vacuum is often quoted, but there are two caveats to it, neither of them encouraging. Firstly, this derived time applies to individuals at rest —that is, not trying to do urgent things in order to stay alive. Secondly, it derives from experiments involving non-abrupt decompression. As Paul W. Fisher reports in the USAF Flight Surgeon’s Guide :
These TUCs are for an individual at rest. Any exercise will reduce the time considerably. For example, usually upon exposure to hypoxia at FL 250 [an altitude of 25,000 ft], an average individual has a TUC of 3 to 5 minutes. The same individual, after performing 10 deep knee bends, will have a TUC in the range of 1 to 1.5 minutes. […] A rapid decompression can reduce the TUC by up to 50 percent caused by the forced exhalation of the lungs during decompression …
Exercise not only increases the consumption of oxygen, it also increases the speed at which blood returns to the lungs and is depleted of its oxygen content. So (extrapolating the extrapolations!) if you’re explosively decompressed while exercising vigorously, it looks like you might end up with less than six seconds of useful consciousness. Which would be disappointing.

Will a person’s blood boil on exposure to vacuum? Yes.
At body temperature, water has a saturated vapour pressure of 47 mmHg. Which means that if the ambient pressure falls below 47 mmHg, the water will evaporate into the gas phase throughout its bulk . Which is the definition of boiling—bubbles forming and expanding within the liquid. The altitude at which the atmospheric pressure drops below 47 mmHg (63,000 ft), and the water in human tissues is in danger of boiling, is called the Armstrong Limit—named for Harry Armstrong , one of the pioneers of aviation medicine.
You’ll find some places on the internet that claim a person’s blood won’t boil, because normal blood pressure (120 mmHg systolic, 80 mmHg diastolic, remember) is higher than that 47 mmHg critical value. In support of this claim, many cite physicist Geoffrey Landis ’s otherwise excellent exposition on explosive decompression :
Your blood is at a higher pressure than the outside environment. A typical blood pressure might be 75/120. The “75” part of this means that between heartbeats, the blood is at a pressure of 75 Torr (equal to about 100 mbar) above the external pressure. If the external pressure drops to zero, at a blood pressure of 75 Torr the boiling point of water is 46 degrees Celsius (115 F). This is well above body temperature of 37 C (98.6 F). Blood won’t boil, because the elastic pressure of the blood vessels keeps it it a pressure high enough that the body temperature is below the boiling point …
Now, the fact that Landis gets the notation for arterial blood pressure reversed (his numbers should be written 120/75) is probably a hint that he’s not entirely at ease with the physiology of blood circulation. What he has forgotten about is the blood that’s not in the arteries , which at any given time amounts to about 90% of the blood volume—flowing through the capillaries, veins and lung blood vessels, all of which normally have pressures well below the critical value. The pressure in the central veins, in particular, is usually only a few millimetres of mercury above ambient. So although Landis writes “No” in answer to the rhetorical question “Would your blood boil?” what he really means is that ten percent of your blood wouldn’t boil, but the rest would. Which certainly seems more like a “Yes” to me.
It’s also sometimes claimed that, while the veins do run at low pressures normally, they will tightly contain any rise in pressure, preventing gas bubbles from forming. But veins are so-called capacitance vessels—they adjust their volume according to the volume of the circulating blood. They will reach their elastic limit if overfilled, but in healthy adults they’re continuously adjusting their volume at low pressure. It’s possible to infuse a litre or more of fluid into the veins of a healthy adult without the venous pressure shifting much over 15 mmHg. So there’s likewise room for a litre or more of gas to form in the venous side of the circulation without causing any major pressure rise in the system. This is a problem, because the amount of gas necessary to cause cardiac arrest in a human is estimated at 3-5 ml/kg—if a few hundred millilitres of gas gets into the heart chambers, it forms a compressible volume that stops the heart propelling liquid when it pumps. The heart continues beating, but it moves no blood.
Another variation on this optimistic theme is that the skin and subcutaneous tissues are tight enough to prevent gas expanding. (Presumably the people who make this claim have never thought seriously about the level of tissue stretchiness implied by a yawn or a clenched fist.) There’s a condition called subcutaneous emphysema , in which gas (usually air from a leaking lung) becomes trapped under the skin. We know that even the relatively low pressures associated with a mechanical ventilator (around 20 mmHg) can squeeze gas out of an already injured lung and into the tissues—which shows that the tissues are unable to immediately generate the necessary counterpressure. Like the veins, the tissues eventually reach an elastic limit and oppose the entry of any further gas, but there is considerable, obvious distension before that happens. So, on exposure to vacuum, the surrounding tissues are not going to be able to prevent the veins expanding.
What happens within the tissues themselves is an interesting question. Dense tissues like tendon and ligament may well be able to contain any tendency for gas bubbles to form within their substance. The loose, soft tissue that lies under the skin (the region affected by subcutaneous emphysema) obviously doesn’t have the structural strength to prevent the spread of gas bubbles once they start forming, but there’s some evidence that it is tightly woven enough to suppress initial bubble formation, for a while.
The second edition of the US Naval Flight Surgeon’s Manual discusses a study in which the hands of volunteers were decompressed to very low pressures. Subcutaneous gas bubbles didn’t form at the Armstrong Limit; they didn’t even form at pressures equivalent to the boiling point of water at skin temperature, which is a little lower than the body’s core temperature. The highest pressure at which gas formation occurred was 20 mmHg; three people were decompressed to 5 mmHg, and all showed gas formation. But the onset of visible gas was delayed —it occurred “suddenly and manifested itself by marked swelling”, but after a lapse of between thirty seconds and over ten minutes of decompression time. Once swelling occurred, it could be rapidly abolished by recompression. But, strikingly, gas was generated much more readily when the hand was decompressed again. So this experiment suggests that it might be initially difficult for gas bubbles to form spontaneously in the tissues, but once they do they can spread rapidly. Other body gases (nitrogen, oxygen, carbon dioxide) will diffuse into these gas bubbles once they form, and will persist as tiny bubbles for some time after recompression has caused the water vapour to collapse back into the liquid phase. These tiny bubbles then form nuclei that will quickly re-expand with water vapour if the tissues are decompressed again.
Unfortunately, the Naval Flight Surgeon’s Manual is a little short on detail. It’s not clear if these volunteers’ hands were still being perfused with blood, or if they’d been isolated by tourniquet, for instance—which would make a significant difference to the tissue pressures. The study was reported in the first edition of NASA’s Bioastronautics Data Book (1964), but was dropped from the second edition of 1973, apparently superceded by more recent experiments which I’ll describe in my next post on this topic. So the pressures and timings in this report are interesting, but may not reflect what happens with total-body decompression.
OTHER THINGS
As if all the foregoing wasn’t enough, a few other problems may occur. If the atmosphere you’re breathing immediately before decompression contains nitrogen, some of that nitrogen will bubble out of solution, in the tissues and blood, as the ambient pressure falls. While this can be a major problem for aviators at altitude, it’s probably a relatively minor problem for those exposed to vacuum, given that water vapour bubbles will be forming. And the second edition of the Bioastronautics Data Book notes that, “The symptoms of decompression sickness are rarely observed during the first few minutes of exposure to low pressure.”
I’ve already mentioned the problem of air trapped in the middle ear. Other areas where air can be trapped, causing pain when the ambient pressure falls, are in the sinuses and under dental fillings. The USN Aerospace Physiologist’s Manual puts the experimental incidence of sinus problems on decompression at 1%, and of dental pain at 0.1%.
Finally, there’s the volume of gas that’s sitting in everyone’s stomach and intestines. This will expand as the ambient pressure falls. On slow decompression it generally causes cramping pain, burping and flatulence. But both the third edition of the USN Flight Surgeon’s Manual and the second edition of the Bioastronautics Data Book note that more rapid expansion of gut gas has the potential to intensely stimulate the vagus nerve, causing a profound fall in heart rate and blood pressure, leading to unconsciousness.
So: You won’t explode, but you may well swell up. You won’t freeze instantly, but your eyes, nose, mouth and airways will experience evaporative cooling. You shouldn’t try to hold your breath. You should breathe out and yawn as the pressure drops, but if decompression is explosive that may not protect your lungs from pressure injury. Your venous blood will boil, and there’s room in your venous circulation to generate enough gas to stop your heart moving blood. The gas in your gut may expand so rapidly it leads to a reflex slowing of your heart and rapid fall in blood pressure. You will have a maximum of 12 seconds of useful consciousness, but if you’re exerting yourself and/or the decompression has been explosive, your period of consciousness may well be considerably shorter.
For Part 2 of this topic, I’m going to look at the data we have from actual vacuum exposure, in humans and animals.
- Go to Part 2
Note: Almost all pressures are quoted in an antique unit of measurement, the millimetre of mercury (mmHg). This is because the most familiar physiological pressure for most people is blood pressure, which is always quoted in millimetres of mercury, and also because a lot of the relevant literature dates back to a time when atmospheric gas pressures were quoted in millimetres of mercury. If you want to convert, the following are equivalents, in round numbers: 1 atmosphere, 1000 millibars, 760 millimetres of mercury, 100 kilopascals, 15 pounds per square inch.
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Click to print (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Pocket (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on Mastodon (Opens in new window)
2 thoughts on “Human Exposure To Vacuum: Part 1”
I seem to recall some discussion of exhalation in Arthur C. Clark’s original 2001 before Bowman decompressed his pod. Clarke, as always, did his research, though I imagine there was also a degree of dramatic license as well.
Yes, by the time of writing 2001 Clarke was aware of some of the animal studies that I’m going to describe in Part 2. He actually wrote an article entitled “A Breath Of Fresh Vacuum” just before 2001 was released, defending the airlock scene and citing references. In it writes, “As long as the main air cavities could be kept open, so that there was no pressure build-up, exposure to vacuum should not cause damage.” Elsewhere (I think in The Lost Worlds of 2001 , but I can’t put my hand on it at the moment), he mentions that Dave Bowman should have breathed out, not held his breath. But Clarke actually wrote about the possibility of people surviving vacuum exposure long before there were any experiments; indeed, before there were any humans in space at all – in the novel Earthlight in 1954, and the short story “Take A Deep Breath” in 1957.
Leave a Reply Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
A discursive blog on various topics of minor interest

How It Works
Expert explanations and breathtaking images ».

World Of Animals
A journey into nature like no other ».

Incredible footage of a NASA test subject being exposed to a space-like vacuum
In 1966 jim le blanc was exposed to a near-vacuum with almost disastrous consequences.
The video above shows the moments when Jim le Blanc was subjected to a space-like vacuum in a NASA testing chamber in 1965. Interestingly, although an accident, it’s one of the only ‘experiments’ into how a human would cope in such conditions, and thus our modern knowledge of the effects rely upon this incident.
Tags: accident , human , incident , NASA , space , survive , what would happen

Publication - Journal Article Recommended methods for conducting human factors experiments on the subjective evaluation of colour rendition

IMAGES
VIDEO
COMMENTS
What Really Happens in a Vacuum. There are a number of things about being in space, in a vacuum, that can cause harm to the human body. The unfortunate space traveler wouldn't be able to hold their breath for long (if at all), because it would cause lung damage. The person would probably remain conscious for several seconds until the blood ...
Subscribe and 🔔 to the BBC 👉 https://bit.ly/BBCYouTubeSubWatch the BBC first on iPlayer 👉 https://bbc.in/iPlayer-Home Brian Cox visits NASA's Space Power ...
In a pair of papers from NASA in 1965 and 1967, researchers found that chimpanzees could survive up to 3.5 minutes in near-vacuum conditions with no apparent cognitive defects, as measured by ...
Unit 731 (Japanese: 731部隊, Hepburn: Nana-san-ichi Butai), [note 1] short for Manchu Detachment 731 and also known as the Kamo Detachment [3]: 198 and the Ishii Unit, [5] was a covert biological and chemical warfare research and development unit of the Imperial Japanese Army that engaged in lethal human experimentation and biological weapons manufacturing during the Second Sino-Japanese War ...
In the absence of pressure, liquid water in our bodies would boil — changing immediately from a liquid to a gas. "In essence, all of your body tissues that contain water will start to expand ...
An unlimited source of vibration sitting in the very vacuum of spacetime. This landmark experiment, first devised by Casimir just after World War II—and only realized 25 years ago—paved the ...
Today, the body at vacuum. The University of Houston presents this series about the machines that make our civilization run, and the people whose ingenuity created them.. I n a famous scene in Stanley Kubrick's "2001 A Space Odyssey", astronaut Dave Bowman ejects himself through open space into an unpressurized airlock of the mother ship — this after the HAL 9000 computer refuses to let him in.
Outer space is often depicted in film as a cold, inhospitable place, where exposure to the perpetual vacuum will make your blood boil and your body burst; alternatively, if neither of those things happen, you're bound to instantly freeze into a human-popsicle. Meanwhile, many of these same films conveniently ignore the slightly more subtle ...
A suite of scientific reports published on 11 June now aims to chart how spaceflight affects space tourists with a wider variety of health histories. The studies show that just a few days in orbit ...
Thermal-Vacuum Human-Rated Testing . Overview | Johnson Space Center (JSC) is the world leader in human-rated testing in a simulated space environment, offering human testing in vacuum, thermal- vacuum, and vibration environments.JSC offers a collection of unique knowledge and experience as to what works well within the hostile environment of space and what does not.
Table of Contents (click to expand) If you are exposed to the vacuum of space, the first thing that will happen is asphyxiation, or lack of oxygen in the bloodstream. You will lose consciousness after about two minutes, and your body will start to swell due to the loss of atmospheric pressure. You will also freeze and be exposed to radiation ...
Joseph Wright's An Experiment on a Bird in the Air Pump (1768) housed at the National Gallery, London. In the mid-17th century Robert Boyle, with the help of Robert Hooke, set about building an air pump and with it a whole system of experimental natural philosophy. Boyle's air pump, or vacuum chamber, created a space for experimentation on ...
McRae 's piece for the exhibition is a speculative vacuum chamber designed to prepare the human body for long-distance space travel. Interplanetary travel will become common, McRae believes, but ...
After Bob reads her message, he can use the newfound knowledge to prepare an experiment that extracts energy from the vacuum — up to the amount injected by Alice. "That information allows Bob, if you want, to time the fluctuations," said Eduardo Martín-Martínez , a theoretical physicist at the University of Waterloo and the Perimeter ...
On STS-37, during one of my flight experiments, the palm restraint in one of the astronaut's gloves came loose and migrated until it punched a hole in the pressure bladder between his thumb and forefinger. ... M. A. Bodin, "Brief Human Vacuum Exposure in Relation to Space Rescue Operations," Journal of the British Interplanetary Society, Vol ...
A human-in-the-loop optimization experiment usually includes an acclimation phase, an optimization phase, stopping when convergence criteria are met, and a separate evaluation of the optimal ...
In Experiment 1, two non-human agents approached each other and two rounds of face-to-face interactive exchanges occurred with co-dependency of exchanged triplet tones 53 (e.g., ...
"It is very unlikely that a human suddenly exposed to a vacuum would have more than 5 to 10 seconds to help himself. If immediate help is at hand, although one's appearance and condition will ...
4. The current record vacuum, made at CERN, is around 1000atoms/cubic cm. For comparison intersteller space (well away from the solar system) is around 1 atom/cc and deep intergalactic, really empty, space is around 0.001 atom/cc. The bits of space we can reach, in Earth orbit, are only about the sort of vacuum you can get in an ordinary lab ...
In the dog experiments, after 90 seconds of exposure to near-vacuum the animal's hearts were still beating, but extremely slowly—about 10 beats per minute. If recompressed at this stage, all the animals survived, but often with transient neurological deficits, presumably from a shower of nitrogen-bubble emboli as the heart started pumping ...
Having exhaled to vacuum, there's now no oxygen at all in the lungs. From the point of view of keeping oxygen circulating in the blood, this is a disaster. Venous blood, returning from the tissues, still contains a considerable residue of oxygen. This is normally topped up by oxygen diffusing from the lungs into the blood.
NASA says, on its official website, that in 1965 an accidental experiment with humans occurred in a vacuum. During a simulation, the costume of an astronaut (whose name is not disclosed) was torn. ... This is impossible because there is not enough pressure in the human body to cause it. However, only three people have died from exposure to the ...
The video above shows the moments when Jim le Blanc was subjected to a space-like vacuum in a NASA testing chamber in 1965. Interestingly, although an accident, it's one of the only 'experiments' into how a human would cope in such conditions, and thus our modern knowledge of the effects rely upon this incident.
Key recommendations include: (1) New work should be motivated by clearly expressed research questions and, when possible, explicit hypotheses that build on the existing body of knowledge, (2) visual stimuli comprising spectral power distributions and visual targets should be deliberately engineered to probe the research questions, (3 ...
Unethical human experimentation is human experimentation that violates the principles of medical ethics.Such practices have included denying patients the right to informed consent, using pseudoscientific frameworks such as race science, and torturing people under the guise of research. Around World War II, Imperial Japan and Nazi Germany carried out brutal experiments on prisoners and ...