The chloride, bromide and iodide precipitates are shown in the photograph:
The chloride precipitate is obviously white, but the other two aren't really very different from each other. You couldn't be sure which you had unless you compared them side-by-side.
All of the precipitates change colour if they are exposed to light - taking on grey or purplish tints.
The absence of a precipitate with fluoride ions doesn't prove anything unless you already know that you must have a halogen present and are simply trying to find out which one. All the absence of a precipitate shows is that you haven't got chloride, bromide or iodide ions present.
The chemistry of the test
The precipitates are the insoluble silver halides - silver chloride, silver bromide or silver iodide.
Ag + (aq) + Cl - (aq) AgCl(s) Ag + (aq) + Br - (aq) AgBr(s) Ag + (aq) + I - (aq) AgI(s)
Silver fluoride is soluble, and so you don't get a precipitate.
Confirming the precipitate using ammonia solution
Carrying out the confirmation
Ammonia solution is added to the precipitates.
Explaining what happens
There is no such thing as an absolutely insoluble ionic compound. A precipitate will only form if the concentrations of the ions in solution in water exceed a certain value - different for every different compound.
This value can be quoted as a solubility product . For the silver halides, the solubility product is given by the expression:
K sp = [Ag + (aq) ][X - (aq) ]
The square brackets have their normal meaning, showing concentrations in mol dm -3 .
If the actual concentrations of the ions in solution produce a value less than the solubility product, you don't get a precipitate. If the product of the concentrations would exceed this value, you do get a precipitate.
Essentially, the product of the ionic concentrations can never be greater than the solubility product value. Enough of the solid is precipitated so that the ionic product is lowered to the value of the solubility product.
Note: If your syllabus says that you need to know about solubility product calculations, you might be interested in my chemistry calculations book where they are explained in detail.
Look at the way the solubility products vary from silver chloride to silver iodide. (You can't quote a solubility product value for silver fluoride because it is too soluble. Solubility products only work with compounds which are very, very sparingly soluble.)
Note: These figures come from the Chemistry Data Book by Stark and Wallace.
You can see that the compounds are all pretty insoluble, but become even less soluble as you go from the chloride to the bromide to the iodide.
What is the ammonia doing?
The ammonia combines with silver ions to produce a complex ion called the diamminesilver(I) ion, [Ag(NH 3 ) 2 ] + . This is a reversible reaction, but the complex is very stable, and the position of equilibrium lies well to the right.
A solution in contact with one of the silver halide precipitates will contain a very small concentration of dissolved silver ions. The effect of adding the ammonia is to lower this concentration still further.
What happens if you multiply this new silver ion concentration by the halide ion concentration? If the answer is less than the solubility product, the precipitate will dissolve.
That happens with the silver chloride, and with the silver bromide if concentrated ammonia is used. The more concentrated ammonia tips the equilibrium even further to the right, lowering the silver ion concentration even more.
The silver iodide is so insoluble that the ammonia won't lower the silver ion concentration enough for the precipitate to dissolve.
An alternative test using concentrated sulphuric acid
If you add concentrated sulphuric acid to a solid sample of one of the halides you get these results:
Note: The chemistry of this test is explained in detail on another page.
The only possible confusion is between a fluoride and a chloride - they would behave identically. You could distinguish between them by dissolving the original solid in water and then testing with silver nitrate solution. The chloride gives a white precipitate; the fluoride doesn't give a precipitate.
Where would you like to go now?
To the Group 7 menu . . .
To the Inorganic Chemistry menu . . .
To Main Menu . . .
© Jim Clark 2002 (last modified March 2022)

Chemistry Dictionary
A complete A-Z dictionary of chemistry terms.
Are you a chemistry student? Visit A-Level Chemistry to download comprehensive revision materials - for UK or international students!

Halide Test
What is halide.
A halide is a dual-phase in which one part is a halogen atom and the other part is an element or radical that is less electronegative or more electropositive than that of halogen to make a fluoride, chloride, bromide, iodide, astatide or hypothetically tennesside compound. Under suitable conditions, the alkali metals combine directly with halogens forming halides by using this general formula
MX (X= F, Cl, Br or I)
All metals present in Group 1 form halides that are white solids at room temperature.
Halide test
We will discuss halide ions (F, Cl, Br, I) tests by using silver nitrate and ammonia.
By Using Silver Nitrate Solution
We need a solution of halide ions. The step is adding a dilute nitric acid to acidify the solution. The nitric acid starts reacting with and removes the other ions present that might form precipitates with silver nitrate.
The second step is to add silver nitrate solution and the following products will be identified by the halide
The formed precipitates of chloride, bromide and iodide precipitates are shown in the photograph below
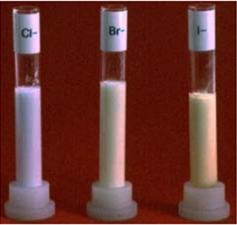
The precipitates of chloride are identified easily but the rest of the two are quite similar to each other. They can be differentiated only in a side-by-side comparison. If the precipitates are exposed to light, all of them will change their colors i.e. grey or purple tints. The fluoride ions will not show any precipitates and the absence of a precipitate ion is unhelpful unless it is well known that a halogen is present otherwise, it will show that the chloride, bromide, or iodide is absent.
The chemistry of the test
The precipitates that are formed are insoluble silver halides: silver chloride, silver bromide or silver iodide. The following equation explains the formation
Ag + (aq) + Cl – (aq) → AgCl (s)
Ag + (aq) + Br – (aq) → AgBr (s)
Ag + (aq) + I – (aq) → AgI( s)
Silver fluoride is soluble so no precipitate is formed.
Ag + (aq) + F – (aq) → Ag + (aq) + F – (aq)
Using ammonia solution to confirm the precipitate
A solution of ammonia is added to the precipitates
Explanation
There are no insoluble ionic compounds. In water, if the concentrations of the ions in solution exceed a certain value, a precipitate will be formed which will be different for every different compound. This value can be considered as a solubility product. The expression for the solubility product of silver halides is given as
K sp = [Ag + ] [X – ]
The square brackets express the molar concentrations having units of mol/L.
Conditions of forming precipitates
- There will be no precipitate formation if the actual concentration of the ions in a solution will produce less value than that of solubility product.
- There will be a precipitate formation if the product of the concentrations would exceed the value of the solubility product.
Necessarily the product of the ionic concentrations is never greater than the solubility product vale. Every time enough solid is precipitated to lower the ionic product to the value of the solubility product. The table given below enlists the solubility products from silver chloride to silver iodide (a solubility product of silver fluoride can’t not be described as it is too soluble).
You will notice that all compounds are very insoluble but become even less soluble as you go down from the chloride to iodide.
The Role of Ammonia
The ammonia comes in contact with silver ions to give a complex ion known as the diamminesilver(I) ion, [Ag(NH 3 ) 2 ] + . This reaction is reversible but the complex is very stable and the equilibrium position lies proficiently to the right. The equation for this reaction is given below:
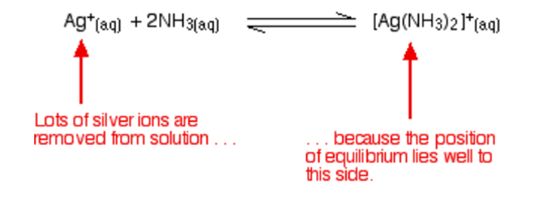
A solution that is in contact with one of the silver halide precipitates will contain a minute concentration of dissolved silver ions. The consequence of adding the ammonia is to lower this concentration still further. If the adjusted silver ion concentration is multiplied by the halide ion concentration is less than the solubility product, some of the precipitates will be dissolved to restore the equilibrium.
If the ammonia is concentrated this will take place with silver chloride and with silver bromide. The more concentrated ammonia pushes the equilibrium even further to the right that lowers the concentration of silver ions even more.
The silver iodide is very insoluble that the ammonia will not lower the silver ion concentration enough for the precipitate to dissolve.
An alternative Test using a concentrated Sulphuric Acid
By adding a concentrated Sulphuric acid to a solid sample of any one of the halide gives the results as follows
The only confusion that can occur between fluoride and chloride is that they behave identically under these conditions. They can be distinguished by dissolving the original solid in water and then testing with a silver nitrate solution. The chloride results in white precipitate while the fluoride produces none.
- https://www.chemguide.co.uk/inorganic/group7/testing.html
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_17%3A_The_Halogens/1Group_17%3A_General_Reactions/Testing_for_Halide_Ions
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alkyl_Halides/Reactivity_of_Alkyl_Halides/Reaction_of_Alkyl_Halides_with_Silver_Nitrate
- https://en.wikipedia.org/wiki/Halide
Please rate these notes
Identify halide ions - chloride, bromide, iodide
In this lesson, we are going to identify halide ions. chloride, bromide, iodide. We study what compounds are used to identify halide ions and what are the observations we can see after halide ions testing. Some halide ions compounds dissolve in water and some form precipitates. According to the physical state and halide ion, we choose different methods and reagents to test halide ion.
What is a halide ion?
Halide ion the anion of halogen atom. The charge of halide ion is -1. F - , Cl - , Br - , I - are the halide ions. These halide ions have some simialar properties and different properties.
All of those halides are in -1 oxidation state. Also these halides can be presence as solid state or solution state. Some of these halide compounds are precipitates. According to the state, we have to change the experimental method to identify the ion.
Identify halide ions in solution state
Here, we are going to discuss about 3 different methods to identify halide ions which exist such as NaCl (aq) , KI (aq) .
Identify chloride ion in solution state

- Add CHCl 3 or CCl 4 and joggle the mixture. It will give colourless layer.
Identify bromide ion in solution state

- Add CHCl 3 or CCl 4 and joggle the mixture. It will give brown/orange globule.
Identify iodide ion in solution state

- Add CHCl 3 or CCl 4 and joggle the mixture. It will give purple globule.

Identify halide ions in solid state
Here, we are going to discuss about 2 different methods to identify halides which exist as solids such as NaCl (s) , KI (s)
Identify chloride ion in solid state compounds
Concentrated sulphuric acid and solid chloride then heat the mixture..

Concentrated sulphuric acid, MnO 2 and solid chloride compound , then heat the mixture.

Chromyl Chloride Test to identify chloride ion
Add K 2 Cr 2 O 7 , concentrated H 2 SO 4 to solid chloride. Then heat the mixture. A red colour vapour CrO 2 Cl 2 is formed.

Add NaOH. CrO 2 Cl 2 and OH - react to give yellow solution CrO 4 2- . Then add CH 3 COOH and Pb(CH 3 COO) 2 . A yellow colour PbCrO 4 precipitated is formed. PbCrO 4(s) solve in dilute HNO 3 .

Identify bromide ion - solid state compounds
Concentrated sulphuric acid and solid bromide compounds then supply heat to the mixture.
It will give brown colour Br 2 gas. H 2 gas also is given.

NaCl (s) gives HBr with concentrated H 3 PO 4 .
When hot concentrated H 3 PO 4 acid is added to solid NaCl, HBr vapour is formed.
Concentrated sulphuric, MnO 2 with solid bromide compounds, then heat the mixture.
It will give brown colour Br 2 gas. MnO 2 is reduced to Mn 2+ ions.
Identify iodide ion in solid state compounds
Concentrated sulphuric acid and solid iodide compound, then heat the mixture..
Iodide ion is oxidized and it will give purple colour I 2 gas. Also hydrogen gas is given.

Concentrated sulphuric acid, MnO 2 and solid iodide compound then heat the mixture.
It will give purple colour I 2 gas.
KI and H 3 PO 4
HI is formed. H 3 PO 4 is not a oxidizing acid.

KI (s) , K 2 Cr 2 O 7 and H 2 SO 4(aq) reaction
Purple colour I 2 is given. Also Cr 3+ is formed. But red CrO 2 Cl 2 vapour is not given.

Iodide ion and Cu 2+ or Fe 3+ reaction
I 2 is released. I 2 can be identified by farina.

Halide ions of alkali metals
All alkali metal halide compounds are high melting crystalline solids. All alkali metal halide compounds are soluble in water exception of LiF. LiF is insoluble in water due to its high lattice energy because of small cation and small anion size. Other halide ions of lithium are soluble in ethanol, acetone, ethyl acetate. LiCl is soluble in pyridine. CsI has also low solubility due to smaller hydration energy of its two ions.
Halide ions of alkali earth metals
Being covalent BeCl 2 is soluble in organic solvents. MgCl 2 , MgBr 2 are soluble in organic solvents. BeCl 2 has low melting point and BaCl 2 has higher melting points. Fluorides of alkali earth metals are sparingly soluble in water. The solubility increases slightly with increase of cation size. All alkali earth metals are ionic and soluble in water except BeCl 2 .
Colours of halide compounds
Some halide compounds have colours. Below all compounds are precipitates.
- PbCl 2(s) : white
- PbBr 2(s) : white cream
- PbI 2(s) : yellow
- AgCl 2(s) : white
- AgBr 2(s) : light yellow
Hydrogen halides
Hydrogen halides are the most useful compounds of halogens. HCl, HBr, HI are strong acids and HF is a weak acid. All of the hydrogen halides are very soluble in water. HCl, HBr, HI are almost completely dissociated in dilute solutions.
Preparing hydrogen halides
Heating a salt containing the halide ion with a nonvolatile acid is the usual way in which HF, HCl, and HBr are obtained in laboratory experiments.
Preparing HCl, HBr and HI

But this method cannot use to prepare HI because HI is not relatively stable than other hydrogen halides.
Preparing HI
prepare a compound containing iodine,then carry out a hydrolysis reaction.

Questions of halide ions
How do you identify sodium chloride and sodium bromide solution.
There are two colourless aqueous solutions without labels. But students have been informed that those two solutions are sodium chloride and sodium bromide. You are advised that. Propose a method to identify these two solutions.
Sodium ion is common in two solutions. So we have to do the testing for chloride ions and bromide ion.
First add dilute nitric acid and then lead nitrate solution. You can notice that a white precipitate and light yellow precipitate form in two solutions. White precipitate is lead chloride. Now we can identify two solutions from comparing colours of two precipitates.
How do you identify sodium nitrite and sodium bromide?
Both sodium bromide and sodium nitrite are white solid compounds at room temperature.
- Add both solids to water separately to see a difference. No you cannot see a change. Both compounds give colourless solutions. So what to do next?
- Add aqueous silver nitrate to colourless solutions. In one flask, a white precipitate is formed.
- Consider existing anions and cations of two solutions.
- In Sodium bromide solution, Na + , Br - , Ag + , NO 3-
- In sodium nitrite, Na + , NO 2- , Ag + , NO 3-
How to test chloride ion in presence of bromide ion?
We can do concentrated sulfuric acid and manganeese dioxide to test chloride ion in the presence of bromide ion. Here how it does.
Manganeese dioxide - MnO 2
Add concentrated sulfuric acid and heat the mixture. You can see a vapour of Br 2 in red brown colour. Then again add concentrated sulfuric acid and MnO 2 . A yello green Cl 2 gas emits.
What are the coloured solutions when add concentrated excess aqueous HCl to transition metal ions?
When we add concentrated excess aqueous HCl to transition metal ions, complexes or coordination compounds are formed. Some 3d metal ions give coloured solutions with concentrated excess aqueous Cl - ions.
3d metal ion complexes when add concentrated excess aqueous Cl - ions
- [FeCl 4 ] 2- (aq) : yellow
- [CoCl 4 ] 2- (aq) : blue
- [NiCl 4 ] 2- (aq) : blue
- [NiCl 4 ] 2- (ethanol) : yellow brown
- [CuCl 4 ] 2- (aq) : yellow
How bromine and iodine are prepared by chlorine gas?
Chlorine is located under iodine and bromine in the electrochemistry series. Therefore reducing of chlorine(Cl 2 ) to chloride(Cl - ) is easier than Br 2 to Br - and I 2 to I - . Bromine is obtained by the oxidation of Br- with chlorine gas in saline water. Iodine is similarly produced by passing chlorine gas through saline water containing I- ions. Cl 2 + 2I - → I 2 + 2Cl - Cl 2 + 2Br - → Br 2 + 2Cl -
sodium bromide with copper presence of sulphuric acid
If you want to release bromine gas, you have to add concentrated sulfuric acid . If you use concentrated sulfuric acid in the presence of copper, copper is oxidized while sulfuric acid is reduced to sulfur dioxide because concentrated sulfuric acid is an oxidizing acid.
How to identify metal halides with potassium ions
All potassium halides ( KF, KCl, KBr, KI ) are soluble in water and give colourless solutions.
- KF - white crystals, soluble in water and HF, but not soluble in alcohol.
- KCl - White crystals, soluble in water, not souble in ethanol.
- KBr - Colorless crystals or white granules or powder, soluble in water, Sparingly soluble in ethanol.
- KI - white solid, soluble in water, Slightly soluble in ethanol.
Only KI is soluble in HF. And KBr and KI are sparingly soluble in ethanol.
Which halide ion, chloride, bromide and iodide is more easy to oxidize?
Iodide ion is easily oxidize to I 2 than bromide to bromine and chloride to chlorine.
Lewis structures
Chemical industries for advanced level chemistry / high school chemistry.

IMAGES
VIDEO