John Dalton
(1766-1844)

Who Was John Dalton?
During John Dalton's early career, he identified the hereditary nature of red-green color blindness. In 1803 he revealed the concept of Dalton’s Law of Partial Pressures. Also in the 1800s, he was the first scientist to explain the behavior of atoms in terms of the measurement of weight.
Early Life and Career
Dalton was born in Eaglesfield, England, on September 6, 1766, to a Quaker family. He had two surviving siblings. Both he and his brother were born color-blind. Dalton's father earned a modest income as a handloom weaver. As a child, Dalton longed for formal education, but his family was very poor. It was clear that he would need to help out with the family finances from a young age.
After attending a Quaker school in his village in Cumberland, when Dalton was just 12 years old he started teaching there. When he was 14, he spent a year working as a farmhand but decided to return to teaching — this time as an assistant at a Quaker boarding school in Kendal. Within four years, the shy young man was made principal of the school. He remained there until 1793, at which time he became a math and philosophy tutor at the New College in Manchester.
While at New College, Dalton joined the Manchester Literary and Philosophical Society. Membership granted Dalton access to laboratory facilities. For one of his first research projects, Dalton pursued his avid interest in meteorology. He started keeping daily logs of the weather, paying special attention to details such as wind velocity and barometric pressure—a habit Dalton would continue all of his life. His research findings on atmospheric pressure were published in his first book, Meteorological Findings , the year he arrived in Manchester.
During his early career as a scientist, Dalton also researched color blindness—a topic with which he was familiar through firsthand experience. Since the condition had affected both him and his brother since birth, Dalton theorized that it must be hereditary. He proved his theory to be true when genetic analysis of his own eye tissue revealed that he was missing the photoreceptor for perceiving the color green. As a result of his contributions to the understanding of red-green color blindness, the condition is still often referred to as "Daltonism."
Dalton's Law
Dalton's interest in atmospheric pressures eventually led him to a closer examination of gases. While studying the nature and chemical makeup of air in the early 1800s, Dalton learned that it was not a chemical solvent, as other scientists had believed. Instead, it was a mechanical system composed of small individual particles that used pressure applied by each gas independently.
Dalton's experiments on gases led to his discovery that the total pressure of a mixture of gases amounted to the sum of the partial pressures that each individual gas exerted while occupying the same space. In 1803 this scientific principle officially came to be known as Dalton's Law of Partial Pressures. Dalton's Law primarily applies to ideal gases rather than real gases, due to the elasticity and low particle volume of molecules in ideal gases. Chemist Humphry Davy was skeptical about Dalton's Law until Dalton explained that the repelling forces previously believed to create pressure only acted between atoms of the same sort and that the atoms within a mixture varied in weight and complexity.
The principle of Dalton's Law can be demonstrated using a simple experiment involving a glass bottle and large bowl of water. When the bottle is submerged under water, the water it contains is displaced, but the bottle isn't empty; it's filled with the invisible gas hydrogen instead. The amount of pressure exerted by the hydrogen can be identified using a chart that lists the pressure of water vapors at different temperatures, also thanks to Dalton's discoveries. This knowledge has many useful practical applications today. For instance, scuba divers use Dalton's principles to gauge how pressure levels at different depths of the ocean will affect the air and nitrogen in their tanks.
During the early 1800s, Dalton also postulated a law of thermal expansion that illustrated the heating and cooling reaction of gases to expansion and compression. He garnered international fame for his additional study using a crudely fashioned dew point hygrometer to determine how temperature impacts the level of atmospheric water vapor.
Atomic Theory
Dalton's fascination with gases gradually led him to formally assert that every form of matter (whether solid, liquid or gas) was also made up of small individual particles. He referred to the Greek philosopher Democritus of Abdera's more abstract theory of matter, which had centuries ago fallen out of fashion, and borrowed the term "atomos" or "atoms" to label the particles. In an article he wrote for the Manchester Literary and Philosophical Society in 1803, Dalton created the first chart of atomic weights.
Seeking to expand on his theory, he readdressed the subject of atomic weight in his book A New System of Chemical Philosophy , published in 1808. In A New System of Chemical Philosophy , Dalton introduced his belief that atoms of different elements could be universally distinguished based on their varying atomic weights. In so doing, he became the first scientist to explain the behavior of atoms in terms of the measurement of weight. He also uncovered the fact that atoms couldn't be created or destroyed.
Dalton's theory additionally examined the compositions of compounds, explaining that the tiny particles (atoms) in a compound were compound atoms. Twenty years later, chemist Amedeo Avogadro would further detail the difference between atoms and compound atoms.
In A New System of Chemical Philosophy , Dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. What that meant was that the molecules of an element are always made up of the same proportions, with the exception of water molecules.
In 1810 Dalton published an appendix to A New System of Chemical Philosophy . In it he elaborated on some of the practical details of his theory: that the atoms within a given element are all exactly the same size and weight, while the atoms of different elements look—and are—different from one other. Dalton eventually composed a table listing the atomic weights of all known elements.
His atomic theories were quickly adopted by the scientific community at large with few objections. "Dalton made atoms scientifically useful," asserted Rajkumari Williamson Jones, a science historian at the University of Manchester Institute of Science and Technology. Nobel Laureate Professor Sir Harry Kroto, noted for co-discovering spherical carbon fullerenes, identified the revolutionary impact of Dalton's discoveries on the field of chemistry: "The crucial step was to write down elements in terms of their atoms...I don't know how they could do chemistry beforehand, it didn't make any sense."
From 1817 to the day he died, Dalton served as president of the Manchester Literary and Philosophical Society, the organization that first granted him access to a laboratory. A practitioner of Quaker modesty, he resisted public recognition; in 1822 he turned down elected membership to the Royal Society. In 1832 he did, however, begrudgingly accept an honorary Doctorate of Science degree from the prestigious Oxford University. Ironically, his graduation gown was red, a color he could not see. Fortunately for him, his color blindness was a convenient excuse for him to override the Quaker rule forbidding its subscribers to wear red.
In 1833 the government granted him a pension, which was doubled in 1836. Dalton was offered another degree, this time a Doctorate of Laws, by Edinburgh University in 1834. As if those honors were insufficient tribute to the revolutionary chemist, in London, a statue was erected in Dalton's honor--also in 1834. "Dalton was very much an icon for Manchester," said Rajkumari Williams Jones. "He is probably the only scientist who got a statue in his lifetime."
In his later life, Dalton continued to teach and lecture at universities throughout the United Kingdom, although it is said that the scientist was an awkward lecturer with a gruff and jarring voice. Throughout his lifetime, Dalton managed to maintain his nearly impeccable reputation as a devout Quaker. He lived a humble, uncomplicated life focusing on his fascination with science, and never married.
In 1837 Dalton had a stroke. He had trouble with his speech for the next year.
Death and Legacy
After suffering a second stroke, Dalton died quietly on the evening of July 26, 1844, at his home in Manchester, England. He was provided a civic funeral and granted full honors. A reported 40,000 people attended the procession, honoring his contributions to science, manufacturing and the nation's commerce.
By finding a way to "weigh atoms," John Dalton's research not only changed the face of chemistry but also initiated its progression into a modern science. The splitting of the atom in the 20th century could most likely not have been accomplished without Dalton laying the foundation of knowledge about the atomic makeup of simple and complex molecules. Dalton's discoveries also allowed for the cost-efficient manufacturing of chemical compounds, since they essentially give manufacturers a recipe for determining the correct chemical proportions in a given compound.
The majority of conclusions that made up Dalton's atomic theory still stand today.
"Now with nanotechnology, atoms are the centerpiece," said Nottingham University Professor of Chemistry David Garner. "Atoms are manipulated directly to make new medicines, semiconductors and plastics." He went on to further explain, "He gave us the first understanding of the nature of materials. Now we can design molecules with a pretty good idea of their properties."
In 2003, on the bicentennial of Dalton's public announcement of his atomic theory, the Manchester Museum held a tribute to the man, his life and his groundbreaking scientific discoveries.
QUICK FACTS
- Name: John Dalton
- Birth Year: 1766
- Birth date: September 6, 1766
- Birth City: Eaglesfield
- Birth Country: United Kingdom
- Gender: Male
- Best Known For: Chemist John Dalton is credited with pioneering modern atomic theory. He was also the first to study color blindness.
- Journalism and Nonfiction
- Science and Medicine
- Education and Academia
- Astrological Sign: Virgo
- John Fletcher's Quaker grammar school
- Death Year: 1844
- Death date: July 26, 1844
- Death City: Manchester
- Death Country: United Kingdom
CITATION INFORMATION
- Article Title: John Dalton Biography
- Author: Biography.com Editors
- Website Name: The Biography.com website
- Url: https://www.biography.com/scientists/john-dalton
- Access Date:
- Publisher: A&E; Television Networks
- Last Updated: May 21, 2021
- Original Published Date: April 2, 2014
- Berzelius' symbols are horrifying. A young student in chemistry might as soon learn Hebrew as make himself acquainted with them.
- We might as well attempt to introduce a new planet into the solar system, or to annihilate one already in existence, as to create or destroy a particle of hydrogen.
- The principal failing in [Sir Humphrey Davy's] character as a philosopher is that he does not smoke.
- I can now enter the lecture room with as little emotion nearly as I can smoke a pipe with you on Sunday or Wednesday evenings.
- Matter, though divisible in an extreme degree, is nevertheless not infinitely divisible. That is, there must be some point beyond which we cannot go in the division of matter... I have chosen the word 'atom' to signify these ultimate particles.
- Will it not be thought remarkable that in 1836 the British chemists are ignorant whether attraction, repulsion or indifference is marked when a mixture of any proportions of azote and oxygen are made.
- In short, [London] is a most surprising place, and worth one's while to see once; but the most disagreeable place on earth for one of a contemplative turn to reside in constantly.
- To ascertain the exact quantity of water in a given quantity of air is, I presume, an object not yet fully attained.
- The cause of rain is now, I consider, no longer an object of doubt.
Watch Next .css-16toot1:after{background-color:#262626;color:#fff;margin-left:1.8rem;margin-top:1.25rem;width:1.5rem;height:0.063rem;content:'';display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;}

Famous British People

Olivia Colman

Richard III

20 Shakespeare Quotes

William Shakespeare

Andy Murray

Stephen Hawking

Gordon Ramsay

Kiefer Sutherland

John Dalton
1766-1844 English Chemist, Physicist and Meteorologist
Early Life and Education
John Dalton was born on September 6, 1766, in Eaglesfield, a small village in Cumberland, England. He was the third of six children born to Joseph Dalton, a weaver, and Deborah Greenup. The Dalton family belonged to the Quaker faith, which emphasized values such as simplicity, hard work, and education. These principles shaped John Dalton's character and his approach to science.
| Engraving of a painting of John Dalton |
Dalton's formal education was limited, ending when he was just 11 years old. However, he continued his education informally under the guidance of John Fletcher, a local Quaker schoolmaster. Fletcher's mentorship was crucial in fostering Dalton's interest in mathematics and science. By the age of 12, Dalton was already teaching at the Quaker school in Eaglesfield. When Fletcher retired in 1781, Dalton and his brother Jonathan took over the school.
Move to Manchester and Early Career
In 1793, Dalton moved to Manchester, a city that was rapidly becoming a center of industrial and scientific activity. He was appointed as a tutor at the New College, a dissenting academy that provided education to students who were excluded from the established universities because of their religious beliefs. Dalton's move to Manchester was pivotal, as it provided him with access to a vibrant intellectual community.
Dalton joined the Manchester Literary and Philosophical Society, where he presented many of his scientific papers. This society played a significant role in Dalton's scientific development, offering him a platform to share his research and collaborate with other scientists.
Contributions to Meteorology
Dalton's initial scientific interests were in meteorology. He was a dedicated observer of the weather and maintained meticulous records of his observations throughout his life. His early work in meteorology culminated in the publication of "Meteorological Observations and Essays" in 1793. This book discussed various meteorological phenomena, including barometric pressure, temperature, and humidity. Dalton's systematic approach to recording and analyzing weather data laid the groundwork for modern meteorology.
One of Dalton's significant contributions to meteorology was his research on the behavior of gases. He formulated Dalton's Law of Partial Pressures, which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gas. This law was a crucial step in understanding the properties of gases and their interactions.
Discovery of Color Blindness
Dalton himself was colorblind, and his condition led him to study the phenomenon scientifically. In 1794, he published a paper titled "Extraordinary facts relating to the vision of colours," which was one of the first systematic studies of color blindness. Dalton theorized that his color blindness was due to a discoloration of the liquid medium in the eyeball, which he believed to be blue. Although this hypothesis was later disproved, Dalton's work was pioneering, and the condition of color blindness is sometimes referred to as Daltonism in his honor.
Development of Atomic Theory
Dalton's most significant contribution to science was his development of modern atomic theory. In 1803, he proposed that all matter is composed of small, indivisible particles called atoms. He suggested that these atoms differ in mass and size depending on the element and that chemical reactions involve the rearrangement of these atoms to form new substances.
Dalton's atomic theory was based on several key postulates:
1. All matter is made up of tiny, indivisible particles called atoms.
2. Atoms of a given element are identical in mass and properties.
3. Atoms of different elements differ in mass and properties.
4. Atoms combine in simple whole-number ratios to form compounds.
5. Atoms are neither created nor destroyed in chemical reactions.
In 1808, Dalton published "A New System of Chemical Philosophy," where he detailed his atomic theory and provided experimental evidence to support his ideas. This work revolutionized the field of chemistry, providing a clear explanation for the laws of chemical combination and laying the foundation for modern chemical science.
Later Life and Recognition
Dalton remained a bachelor throughout his life, dedicating himself to his scientific pursuits and teaching. He lived modestly, in accordance with his Quaker beliefs, and was known for his frugal lifestyle. Despite his significant contributions to science, Dalton never sought personal wealth or luxury.
Throughout his career, Dalton received numerous honors and recognitions. He was elected a Fellow of the Royal Society in 1822, acknowledging his contributions to science. In 1830, he was elected to the French Academy of Sciences, and in 1834, he received an honorary degree from the University of Edinburgh.
Dalton continued his scientific work until the end of his life. He suffered a stroke in 1837, which left him with a speech impairment, but he continued to conduct experiments and write papers. John Dalton passed away on July 27, 1844, in Manchester. His death was widely mourned, and his funeral procession was attended by thousands of people.
Legacy and Impact
John Dalton's contributions to science are profound and enduring. His atomic theory fundamentally changed the understanding of chemistry, providing a clear framework for explaining chemical reactions and the nature of matter. Dalton's meticulous approach to scientific research set high standards for future scientists, emphasizing the importance of systematic observation and experimentation.
Dalton's work on color blindness opened new avenues in the study of vision and perception. His dedication to meteorology provided invaluable data that has benefited the field for generations. His influence extends beyond his lifetime, as his theories and findings remain fundamental to the study of chemistry and physics.
Dalton's legacy is honored through numerous awards, statues, and institutions named after him. The statue of John Dalton in Manchester and the John Dalton Building at Manchester Metropolitan University are testaments to his lasting impact. His life and work continue to inspire scientists and scholars, exemplifying the power of curiosity, perseverance, and the pursuit of knowledge.
In summary, John Dalton's contributions to science have left an indelible mark on the scientific community. His pioneering work in atomic theory, meteorology, and color blindness continues to influence and inspire generations of scientists and researchers. Dalton's legacy is a testament to the enduring power of scientific inquiry and the pursuit of knowledge.

- Scientific Biographies
John Dalton
The theory of atomism, proposed by Dalton in the early 19th century and derived from meteorological studies, is the foundation for our modern concept of the atom.

Although a schoolteacher, a meteorologist, and an expert on color blindness, John Dalton is best known for his pioneering theory of atomism. He also developed methods to calculate atomic weights and structures and formulated the law of partial pressures.
Dalton (1766–1844) was born into a modest Quaker family in Cumberland, England, and for most of his life—beginning in his village school at the age of 12—earned his living as a teacher and public lecturer. After teaching for 10 years at a Quaker boarding school in Kendal, he moved on to a teaching position in the burgeoning city of Manchester.
There he joined the Manchester Literary and Philosophical Society, which provided him with a stimulating intellectual environment and laboratory facilities. The first paper he delivered before the society was on color blindness, which afflicted him and is sometimes still called Daltonism.
Theories of Atomism and the Law of Partial Pressures
Dalton arrived at his view of atomism by way of meteorology, in which he was seriously interested for a long period: he kept daily weather records from 1787 until his death, his first book was Meteorological Observations (1793), and he read a series of papers on meteorological topics before the Literary and Philosophical Society between 1799 and 1801.
The papers contained Dalton’s independent statement of Charles’s law (see Joseph Louis Gay-Lussac ): “all elastic fluids expand the same quantity by heat.” He also clarified what he had pointed out in Meteorological Observations —that the air is not a vast chemical solvent as Antoine-Laurent Lavoisier and his followers had thought, but a mechanical system, where the pressure exerted by each gas in a mixture is independent of the pressure exerted by the other gases, and where the total pressure is the sum of the pressures of each gas.

In explaining the law of partial pressures to skeptical chemists of the day—including Humphry Davy —Dalton claimed that the forces of repulsion thought to cause pressure acted only between atoms of the same kind and that the atoms in a mixture were indeed different in weight and “complexity.”
Experiments on Atomic Weights and Structures
He proceeded to calculate atomic weights from percentage compositions of compounds, using an arbitrary system to determine the likely atomic structure of each compound. If there are two elements that can combine, their combinations will occur in a set sequence. The first compound will have one atom of A and one of B; the next, one atom of A and two atoms of B; the next, two atoms of A and one of B; and so on.
Hence, water is HO. Dalton also came to believe that the particles in different gases had different volumes and surrounds of caloric, thus explaining why a mixture of gases—as in the atmosphere—would not simply layer out but was kept in constant motion. Dalton consolidated his theories in his New System of Chemical Philosophy (1808–1827).
As a Quaker, Dalton led a modest existence, although he received many honors later in life. In Manchester more than 40,000 people marched in his funeral procession.
Featured image: Portrait print of Dr. John Dalton, F.R.S. , 1834. Science History Institute
Browse more biographies

Hideki Shirakawa

Herbert Henry Dow

Maxine Singer
Copy the above HTML to republish this content. We have formatted the material to follow our guidelines, which include our credit requirements. Please review our full list of guidelines for more information. By republishing this content, you agree to our republication requirements.
Registration
| Country: |
Biography of John Dalton
Early life and education, career and scientific contributions, atomic theory and later life.
John Dalton, an English chemist and physicist, is known for his contributions to the development of atomic theory. He established the law of multiple proportions in 1803 and introduced the concept of atomic weight. Dalton was the first to determine the atomic weights of several elements and discovered the gas laws that were named after him. He also described the visual defect known as color blindness, later named Daltonism, that he himself suffered from.
John Dalton was born on September 6, 1766, in a poor family in the small English village of Eaglesfield. At the age of thirteen, he completed his education at a local school and became an assistant teacher. In the autumn of 1781, he became a mathematics teacher in Kendal. Dalton started his scientific research in 1787, focusing on the observation and experimental study of air. He also developed an interest in mathematics and independently worked on new mathematical problems and solutions. Dalton wrote his first scientific papers in this field. After four years, he became the headmaster of the school. During this time, Dalton formed a close relationship with Dr. Charles Hutton, the editor of several journals of the Royal Military Academy. Dalton became one of the regular contributors to these almanacs and received several prestigious awards for his contributions to the development of mathematics and philosophy.
In 1793, Dalton moved to Manchester, where he taught at the New College. He brought with him the manuscript "Meteorological Observations and Essays," in which he analyzed the processes of cloud formation, evaporation, distribution of atmospheric precipitation, and the morning north winds, along with descriptions of barometers, thermometers, hygrometers, and other instruments. Dalton became a member of the Literary and Philosophical Society in 1794 and served as its secretary in 1800. He was elected as the vice-president in May 1808 and served as the president from 1817 until his death. In 1794, Dalton presented a lecture on color blindness, a visual defect now known as Daltonism. In 1799, he left the New College and became the highest-paid private tutor in Manchester, teaching only a few hours a day in wealthy families and devoting the rest of his time to scientific research. Dalton's attention was drawn to gases and gas mixtures, leading him to make several fundamental discoveries, including the law of uniform expansion of gases upon heating in 1802, the law of multiple proportions in 1803, and the phenomenon of polymerism using examples of ethylene and butylene.
On September 6, 1803, Dalton recorded the first table of atomic weights in his laboratory journal. He first mentioned the atomic theory in his paper "On the Absorption of Gases by Water and Other Liquids," presented on October 21, 1803, at the Manchester Literary and Philosophical Society. From December 1803 to May 1804, Dalton delivered a series of lectures on relative atomic weights at the Royal Institution in London. He further developed his atomic theory in his book "A New System of Chemical Philosophy," published in 1808. The book emphasized two key points: all chemical reactions are the result of the combination or division of atoms, and atoms of different elements have different weights.
In 1816, Dalton was elected a corresponding member of the Paris Academy of Sciences. The following year, he became the president of the Manchester Society, and in 1818, the British government appointed him as a scientific expert for an expedition led by Sir John Ross. However, Dalton preferred the quiet work in his laboratory and declined the opportunity. He continued his research on atomic weights.
In 1822, Dalton became a member of the Royal Society. Shortly after, he traveled to France, where the Paris Academy of Sciences appointed him to its honorary council, recognizing his outstanding contributions to the field of science.
In 1826, Dalton was awarded the Royal Society's highest honor, the Copley Medal, for his discoveries in the fields of chemistry and physics, primarily for his development of atomic theory. He was elected as an honorary member of the Berlin Academy of Sciences, the Moscow Society of Naturalists, and the Munich Academy.
In 1832, Dalton received the highest distinction from the University of Oxford, as he was awarded an honorary Doctor of Laws degree. Among the scientists of that time, only Michael Faraday received a similar honor.
In 1833, Dalton was granted a pension, and the decision of the government was announced at a ceremonial session at the University of Cambridge.
Despite his advanced age, Dalton continued to work diligently and present papers. However, with old age, he began to suffer from frequent illnesses and found it increasingly difficult to work. On July 27, 1844, Dalton passed away.
| | | |
| | | |
© BIOGRAPHS
Biography Online

John Dalton Biography

1. Small particles called atoms exist and compose all matter; 2. They are indivisible and indestructible; 3. Atoms of the same chemical element have the same chemical properties and do not transmute or change into different elements.
Dalton also pursued a wide range of interests from English grammar and physics to meteorology and colour blindness – a subject where he made ground-breaking discoveries.
Dalton was born in Eaglesfield near Cockermouth in the north Lake District in England. His family were Quaker and relatively poor – despite his father’s position as a teacher. At the age of 10, Dalton worked in service to a wealthy Quaker and received no more formal education. However, he continued to teach himself – proving highly intelligent and determined to learn. By the age of 15, he was fluent in Latin and was working as a teacher in a Quaker school in Kendal.
Growing up in Eaglesfield, he was inspired by Elihu Robinson a prominent Quaker and meteorologist. This sparked a lifelong interest in meteorology and for 57 years from 1787 to his death in 1844; he entered more than 200,000 observations on meteorology. In his spare time, he began writing scientific essays for journals and developing his own observations.
Whilst in the Lake District he would combine his love for the region’s hills with his passion for meteorology. Using a homemade barometer, he would climb mountains to ascertain their height. In an era before maps, Dalton became an authority on the region’s mountains and fells. Even when working away from the Lake District, he would return at least once per year to climb the fells.
Despite his precocious intellect and enthusiasm for studying, he did not pursue a career in law or medicine because at the time (dissenters (non-Anglicans) were barred from studying at English universities. However, he studied science informally under John Gough. In 1793, he was appointed to be a teacher of maths and natural philosophy at “New College” Manchester – a new dissenting university. He stayed there for seven years, before returning to his role as a private tutor. Around this time, his first work was published “Meteorological Observations and Essays.” (1793)
Shortly after his arrival in Manchester, Dalton studied the condition of colour blindness – something both he and his brother suffered from. Dalton postulated that colour blindness stemmed from discolouration of the liquid medium in the eyeball. This later proved to be inaccurate. However, Dalton’s careful, thorough and methodical enquiries into the subject were pioneering and became a template for the subject. Dalton was such an influential figure that Daltonism became a common term for colour blindness.
In 1800, he pursued experiments on the effect of the thermal expansion of gases. His experiments and conclusions were presented to The Manchester Literary and Philosophical Society and it showed that different liquids all showed the same variation in vapour pressure from the same variation in temperature. In 1802, he published “On the expansion of elastic fluids by heat” This showed that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. This became known as “Dalton’s Law of partial pressure”
Atomic Chemistry
Dalton’s most influential work by far was his investigations into the atomic structure of elements. Dalton deduced that elements are made of extremely small particles, called atoms. The atoms are individual to each element but can be combined with other elements to form compounds which are multiples of the original atomic elements. He also stated atoms cannot be divided, created or destroyed – only in chemical reactions we see a change in how atoms combine.
For example, water is a combination of atoms from Hydrogen and Oxygen. H2O, (H2O, NH3)
Dalton also tried to predict the relative atomic weights of different elements, using hydrogen as the base weight of 1. He proposed a list of atomic weights – some of which proved to be correct like CO and CO2 (carbon dioxide) but some which were not correct, such as water, he proposed HO rather than H2O.
Atomic theory was not entirely new. The theory of the atom stretched back into classical time, and other scientists, such as his uncle Bryan Higgins were working on a caloric model for atoms. However, Dalton’s breakthrough was to provide a method for calculating relative atomic weights for chemical elements. Dalton’s real achievement was to study the atom and chemical compounds in a quantitative fashion.
In 1804, Dalton first proposed his law of multiple proportions. It was published in 1808 in “New System of Chemical Philosophy” The law states:
“If two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers.”
Dalton was criticised by Sir Humphrey Davy for being a ‘very coarse experimenter” Dalton didn’t always use the most exact instruments which were available. He preferred to trust in his skill, intuition and often ended up getting the results he had wanted. However, many of his experiments have been validated and despite the inadequacies of his equipment, he has proved successful from many perspectives.
Dalton lived a modest personal life, remaining unmarried and mostly devoted to his work. Despite his international reputation, he remained quite poor, living a simple life. James Forbes, a Scottish physicist, wrote in glowing terms after meeting Dalton in Manchester:
“He (Dalton) is generally styled by continental writers the Father of Modern Chemistry, and is one of the eight associates of the Institute. Yet this man between sixty and seventy is earning, as I had a peculiar satisfaction in seeing with my own eyes, a penurious existence by teaching boys the elements of mathematics, with which he is so totally occupied, that he can hardly snatch a moment for the prosecution of discoveries which have already put his name on a level with the courtly and courted Davy. But the remarkable thing is that this simple and firm-minded man preserves all the original simplicity and equanimity of his mind, and calmly leaves his fame, like Bacon, to other nations and future ages.” Life and letters of James David Forbes p. 450
Dalton continued to pursue experiments, and make meteorological observations and teach until his death. In later years, he taught the young chemist James Prescott Joule, who later became well-known for his investigations into heat. He lived in a home with Rev W. Johns and his wife. In 1837, he suffered a minor stroke, which led to a speech impairment. In 1844, he had a more devastating stroke and passed away within a day on 27 July 1844. Dalton’s reputation as a well-respected scientist led to a civil funeral with full honours. More than 40,000 people visited his coffin, while it was lying in Manchester Town Hall.
Citation: Pettinger, Tejvan . “John Dalton Biography”, Oxford, UK. www.biographyonline.net Published 27 April 2019.
100 Scientists Who Shaped World History

100 Scientists Who Shaped World History at Amazon

- Discoveries that changed the world
- Art History
- U.S. History
John Dalton
| John Dalton | |
|---|---|
| Chemist | |
| Specialty | Chemistry, Physics, Meteorology |
| Born | Sep. 6, 1766 Eaglesfield, Cumberland, England |
| Died | July 27, 1844 (at age 77) Manchester, England |
| Nationality | English |
John Dalton was an English scientist who was born in Eaglesfield, near Cockermouth, in the northwest region of England in 1766. Despite the fact that he was born into a poor family of weavers, Dalton embraced education and proved to be a very precocious child.
Dalton’s Early Years
Dalton distinguished himself academically at a very early age by achieving the remarkable accomplishment of being appointed a teacher in the local school when he was only 12 years old. He moved to nearby Kendal when he was 15, taking a post as principal of the Quaker school in that town.
Even at such a young age, Dalton was very diligent in keeping diaries. From these diaries, we know that he had developed an interest in scientific and mathematical subjects. In fact, he had already begun to study Isaac Newton’s great work, Principia . Even more remarkable, he was studying it in Latin, the language in which it had been written.
Life in Manchester
When Dalton was 27, he moved to the city of Manchester, where he continued working as a teacher at New College. He became a prolific writer on a wide range of topics ranging from medical issues to grammar to meteorology. Dalton was color blind, and this led him to devote much valuable research into understanding the condition. This work was so influential that the condition became known as Daltonism. He correctly surmised that the condition must be hereditary.
Manchester Literary and Philosophical Society
John Dalton became a member of the Manchester Literary and Philosophical Society, and his membership gave him permission to use the laboratories to conduct experiments. It was during this period that he formulated what became known as Dalton’s Law, which states that the total pressure exerted by a mixture of gases is equal to the sum of the pressure exerted by each gas in the mixture.
In 1808, Dalton published a tome of more than 900 pages, which he entitled A New System of Chemical Philosophy . In a short section of this book, he became the first scientist to identify the true nature of atoms, and his conclusions were pretty much the same as how we understand atoms today.
Working with Atoms
Two years later, Dalton published an appendix to the book, where he explained further how the atoms that made up any particular element all had the same weight and size. The weight and size of the atoms of different elements are different from each other. Dalton’s work can accurately be described as the foundation of modern chemistry.
Honors and Legacy
John Dalton was a retiring individual who was offered many prestigious awards, most of which he declined. However, in 1817, he accepted the position of president of the Manchester Literary and Philosophical Society, and he remained in that post until his death. He declined an offer from the Royal Society to become a member. When Oxford University wanted to offer him an honorary Doctorate of Science in 1832, he reluctantly accepted it.
His contribution to science and education was widely recognized during his lifetime, something that eluded many other scientists. He was granted a pension by the government of the day in 1833. Three years later, the government doubled the value of his pension. A statue of Dalton was erected in London in 1834, which was an impressive tribute to a man who was still alive.
Dalton died at his Manchester home in 1844, and the city granted him a full civic funeral. In a significant testament to his fame and the prestige he had established, the attendance at his funeral was estimated at an extraordinary 40,000 people.
Newest Additions
- Malala Yousafzai
- Greta Thunberg
- Frederick Douglass
- Wangari Maathai
Copyright © 2020 · Totallyhistory.com · All Rights Reserved. | Terms of Use | Privacy Policy | Contact Us

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

John Dalton summary

John Dalton , (born Sept. 5 or 6, 1766, Eaglesfield, Cumberland, Eng.—died July 27, 1844, Manchester), British chemist and physicist. He spent most of his life in private teaching and research. His work on gas es led him to state Dalton’s law ( see gas laws). He devised a system of chemical symbols, ascertained the relative weights of atoms, and arranged them into a table. His masterpiece of synthesis was the atomic theory—the theory that each element is composed of tiny, indestructible particles called atom s that are all alike and have the same atomic weight —which elevated chemistry to a quantitative science. He was also the first to describe colour blindness (1794), and his lifelong meteorological journal contains more than 200,000 observations. He is remembered as one of the fathers of modern physical science.


John Dalton’s 10 Major Contributions And Accomplishments
Born in September 1766 , John Dalton was an English scientist who did pioneering work in the fields of chemistry and meteorology. He was the first to publish a paper on colour blindness and also provided great new insights into the nature of gases. He was renowned during his life though the enormous nature of his contribution was realized with further advancements in science. Here are the 10 major accomplishments of John Dalton including his remarkable contribution to chemistry and meteorology.
#1 HE MADE SEVERAL REMARKABLE METEOROLOGICAL OBSERVATIONS
Dalton’s first major achievements were in meteorology, the scientific study of atmosphere. In 1793, Meteorological Observations and Essays became his first published work. It asserted for the first time that water vapour existed independently in air and didn’t combine chemically with other atmospheric gases. It also contained his study of aurora borealis which detected the magnetic relation of the phenomenon and concluded its light to be of purely electrical origin. Dalton made important contributions to meteorology throughout his scientific career and was called the Father of Meteorology by John Frederic Daniell .

#2 He PUBLISHED THE FIRST EVER PAPER ON COLOUR BLINDNESS
John Dalton was colour blind and so was his elder brother Jonathan Dalton. In his 1794 paper “Extraordinary facts relating to the vision of colours” Dalton described the defect he had discovered in his own and his brother’s vision. This paper was the first publication on colour blindness . Though Dalton correctly recognized that the deficiency was hereditary, his theory regarding it was incorrect. Still colour blindness is sometimes referred to as Daltonism as he was the first scientist to thoroughly investigate the defect .
#3 JOHN DALTON DID PIONEERING WORK IN HYDROLOGY
Dalton’s 1799 paper proposed after research and estimated calculations that the quantity of rain and dew are equal to the quantity of water carried off by evaporation and by the rivers . It also contained the earliest definition of the ‘dew-point’ and settled for all practically purposes the controversy over the origin of springs by his conclusion that they are fed by rain. This paper was an important step in the development of quantitative hydrological cycles . Due to John Dalton’s contribution, the Dalton Medal is given to hydrologists by the European Geophysical Society for distinguished research in the field.

#4 HE PROVIDED GREAT NEW INSIGHTS INTO THE NATURE OF GASES
In 1802, John Dalton’s ground-breaking research, which provided great new insights on the nature of gases, was published. In it he noted correctly that all gases could be liquefied provided their temperature was sufficiently low and pressure sufficiently high; and that all gases expand the same quantity by heat . He also came up with what is known as Dalton’s law of evaporation . It states that the rate of evaporation is proportional to the difference between the saturation vapour pressure at water temperature and the actual vapour pressure in air.
#5 HE OBSERVED WHAT IS KNOWN AS DALTON’S LAW OF PARTIAL PRESSURES
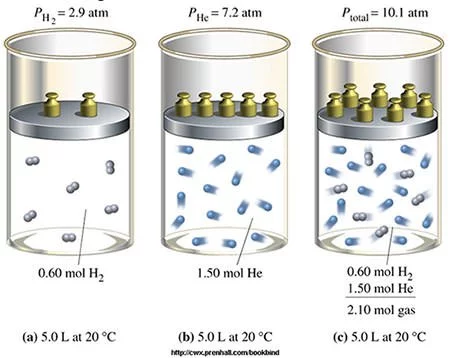
In 1801, John Dalton found that volume of all gases he studied increased proportionally with rise in temperature when pressure was held constant (VαT at constant P) . The law however bears the name of French scientist Jacques Charles , who had formulated it earlier but never published the results. In 1803, Dalton published his Law of Partial Pressures , which states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases . Also known as Dalton’s Law , it is commonly applied in looking at the pressure of a closed container of gas and water.

#6 HIS LAW OF MULTIPLE PROPORTIONS IS ONE OF THE BASIC LAWS OF STOICHIOMETRY
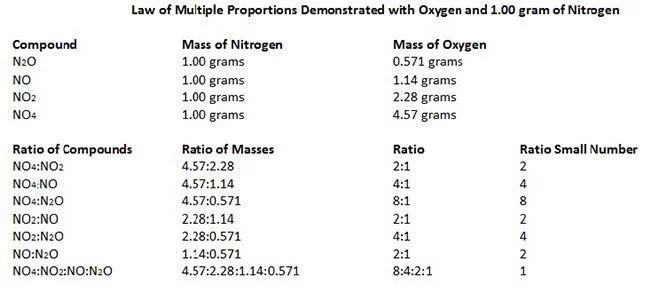
Two important laws dealing with chemical reactions emerged near the end of the 18th century – Antoine Lavoisier’s law of conservation of mass and Joseph Proust’s law of definite proportions . Through the study of these laws and experimentation John Dalton developed his law of multiple proportions , which states that if two elements can be combined to form a number of possible compounds, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers. The three laws mentioned above form the basis of Stoichiometry , i.e. the calculation of relative quantities of reactants and products in chemical reactions.
#7 HE PROPOSED THE FIRST TRULY SCIENTIFIC ATOMIC THEORY
Dalton’s law of multiple proportions, which he announced in 1803 , became the basis for his famous Atomic Theory which he proposed later that year . The 5 main points of Dalton’s atomic theory are: elements are made of extremely small particles called atoms; atoms of a particular element are identical; atoms cannot be created, destroyed or split; atoms of different elements combine in simple whole-number ratios to form chemical compounds; and in a chemical reaction, atoms link to one another, or separate from one another. Dalton’s theory was the first truly scientific theory of the atom reached through analysis and experimentation.
#8 HiS ATOMIC THEORY LAID THE FOUNDATION OF MODERN CHEMISTRY
Though later research found that atoms of the same element are not necessarily identical as they can have different masses (isotopes) and that atoms can be split in nuclear reactions; Dalton’s atomic theory holds good in several aspects even today and it remains valid for chemical reactions . Also Dalton’s theory laid the foundation of modern chemistry and the basis on which future scientists made numerous other highly significant discoveries.
#9 DALTON WAS THE FIRST TO CALCULATE RELATIVE ATOMIC WEIGHTS
On the basis of his atomic theory, John Dalton calculated the first relative weights of atoms. He estimated the atomic weights according to the mass ratios in which they combined; with the hydrogen atom taken as unity. He proceeded to print the first published table of relative atomic weights . Published in 1803 , his first list contained only 6 elements. This was followed by a 20 elements list in 1808 and a 36 element list in 1827. In the long run atomic weights would provide the key means of organizing elements into the periodic table .

#10 HE RECEIVED SEVERAL HONOURS INCLUDING THE ROYAL MEDAL
John Dalton served as president of the Manchester Literary and Philosophical society (the “Lit & Phil”) from 1817 till his death. In 1822, he was made a fellow of the Royal Society of London and in 1826 he was awarded the Society’s Royal Medal for his Atomic Theory. In 1830, Dalton was elected one of only eight foreign members of the French Academy of Sciences and in 1834 he was elected a Foreign Honorary Member of the American Academy of Arts and Sciences . Also Francis Leggatt Chantrey’s statue of him made John Dalton probably the only scientist who got a statue in his lifetime .
3 thoughts on “John Dalton’s 10 Major Contributions And Accomplishments”
He was a great man His research were wonderful and as the matter of fact the basis in chemistry
Super Dalton
wonderful work done by Dalton,,
Leave a Comment Cancel reply
Privacy overview.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
John Dalton

John Dalton ( September 6 , 1766 July 27 , 1844 ) was a British chemist and physicist, born at Eaglesfield, near Cockermouth in Cumberland. He is most well known for his advocacy of the atomic theory.
Dalton received his early education from his father and from John Fletcher, a teacher of the Quaker school at Cumberland, on whose retirement in 1778 he himself started teaching. This youthful venture was not successful, the amount he received in fees being only about five shillings a week, and after two years he took to farm work. But he had received some instruction in mathematics from a distant relative, Elihu Robinson, and in 1781 he left his native village to become assistant to his cousin George Bewley, who kept a school at Kendal. There he passed the next twelve years, becoming in 1785, through the retirement of his cousin, joint manager of the school with his elder brother Jonathan.
About 1790 he seems to have thought of taking up law or medicine, but his projects met with no encouragement from his relatives and he remained at Kendal until, in the spring of 1793, he moved to Manchester. Mainly through John Gough, a blind philosopher to whose aid he owed much of his scientific knowledge, he was appointed teacher of mathematics and natural philosophy at the Manchester Academy. He remained in that position until the relocation of the college to York in 1803, when he became a public and private teacher of mathematics and chemistry. Among his pupils were: Eaton Hodgkinson and James Prescott Joule.
Meteorology, vision and miscellany
During his years in Kendal, Dalton had contributed solutions of problems and questions on various subjects to the Gentlemen's and Ladies' Diaries, and in 1787 he began to keep a meteorological diary in which, during the succeeding fifteen years, he entered more than 200,000 observations. His first separate publication was Meteorological Observations and Essays (1793), which contained the germs of several of his later discoveries. However, in spite of the originality of his treatment, the book met with only a limited sale.
Another work by him, Elements of English Grammar, was published in 1801. In 1794 he was elected a member of the Manchester Literary and Philosophical Society, the Lit & Phil, and a few weeks after election he communicated his first paper on Extraordinary facts relating to the vision of colours, in which he gave the earliest account of the optical peculiarity known as Daltonism or colour blindness, and summed up its characteristics as observed in himself and others, including his brother. Besides the blue and purple of the spectrum he was able to recognize only one colour, yellow, or, as he says in his paper, that part of the image which others call red appears to me little more than a shade or defect of light. After that the orange, yellow and green seem one colour which descends pretty uniformly from an intense to a rare yellow, making what I should call different shades of yellow.
This paper was followed by many others on diverse topics on rain and dew and the origin of springs, on heat, the colour of the sky, steam, the auxiliary verbs and participles of the English language and the reflection and refraction of light.
Atomic theory
In 1800 he became a secretary of the Lit & Phil, and in the following year he presented the important paper or series of papers, entitled Experimental Essays on the constitution of mixed gases; on the pressure of steam and other vapours at different temperatures, both in a vacuum and in air; on evaporation; and on the thermal expansion of gases.
The second of these essays opens with the striking remark,
There can scarcely be a doubt entertained respecting the reducibility of all elastic fluids of whatever kind, into liquids; and we ought not to despair of effecting it in low temperatures and by strong pressures exerted upon the unmixed gases further.
After describing experiments to ascertain the pressure of steam at various points between 0 ° and 100°C (32° and 212°F), he concluded from observations on the vapour pressure of six different liquids, that the variation of vapour pressure for all liquids is equivalent, for the same variation of temperature, reckoning from vapour of any given pressure.
In the fourth essay he remarks,
"I see no sufficient reason why we may not conclude that all elastic fluids under the same pressure expand equally by heat and that for any given expansion of mercury, the corresponding expansion of air is proportionally something less, the higher the temperature. It seems, therefore, that general laws respecting the absolute quantity and the nature of heat are more likely to be derived from elastic fluids than from other substances."
He thus enunciated Gay-Lussac's law, stated some months later by Joseph Louis Gay-Lussac. In the two or three years following the reading of these essays, he published several papers on similar topics, that on the absorption of gases by water and other liquids (1803), containing his law of partial pressures.
The most important of all Dalton's investigations are those concerned with the atomic theory in chemistry, with which his name is inseparably associated. It has been proposed that this theory was suggested to him either by researches on ethylene (olefiant gas) and methane (carburetted hydrogen) or by analysis of nitrous oxide (protoxide of azote) and nitrogen dioxide (deutoxide of azote), both views resting on the authority of Thomas Thomson. However, a study of Dalton's own laboratory notebooks, discovered in the rooms of the Lit & Phil[1], concluded that so far from Dalton being led to the idea, that chemical combination consists in the interaction of atoms of definite and characteristic weight, by his search for an explanation of the law of multiple proportions, the idea of atomic structure arose in his mind as a purely physical concept, forced upon him by study of the physical properties of the atmosphere and other gases. The first published indications of this idea are to be found at the end of his paper on the absorption of gases already mentioned, which was read on October 21, 1803 though not published till 1805. Here he says:
"Why does not water admit its bulk of every kind of gas alike? This question I have duly considered, and though I am not able to satisfy myself completely I am nearly persuaded that the circumstance depends on the weight and number of the ultimate particles of the several gases."
He proceeds to give what has been quoted as his first table of atomic weights, but in his laboratory notebooks[2] there is an earlier one dated 1803 in which he sets out the relative weights of the atoms of a number of substances, derived from analysis of water, ammonia, carbon dioxide, etc. by chemists of the time.
It appears, then, that confronted with the problem of calculating the relative diameter of the atoms of which, he was convinced, all gases were made, he used the results of chemical analysis. Assisted by the assumption that combination always takes place in the simplest possible way, he thus arrived at the idea that chemical combination takes place between particles of different weights, and this it was which differentiated his theory from the historic speculations of the Greeks.
The extension of this idea to substances in general necessarily led him to the law of multiple proportions, and the comparison with experiment brilliantly confirmed his deduction[3]. It may be noted that in a paper on the proportion of the gases or elastic fluids constituting the atmosphere, read by him in November 1802, the law of multiple proportions appears to be anticipated in the words: The elements of oxygen may combine with a certain portion of nitrous gas or with twice that portion, but with no intermediate quantity, but there is reason to suspect that this sentence was added some time after the reading of the paper, which was not published till 1805.
Many of Dalton's ideas were acquired from other chemists at the time, such as Antoine Lavoisier and William Higgins. However, he was the first to put the ideas into a universal atomic theory, which was undoubtedly his greatest achievement.
Four main points of Dalton's Atomic Theory 1. Each element is composed of tiny particles called atoms. 2. The atoms of a given element are identical (and have identical mass). Corollary- Atoms of differednt elements are different (and have different masses). 3. A chemical compound has the same relative nmber of different atoms 4. A chemical reaction involves rearrangement of atoms.
Later years
Dalton communicated his atomic theory to Thomson who, by consent, included an outline of it in the third edition of his System of Chemistry (1807), and Dalton gave a further account of it in the first part of the first volume of his New System of Chemical Philosophy (1808). The second part of this volume appeared in 1810, but the first part of the second volume was not issued till 1827, though the printing of it began in 1817. This delay is not explained by any excess of care in preparation, for much of the matter was out of date and the appendix giving the author's latest views is the only portion of special interest. The second part of vol. ii. never appeared.
Dalton was president of the Lit & Phil from 1817 until his death, contributing 116 memoirs. Of these the earlier are the most important. In one of them, read in 1814, he explains the principles of volumetric analysis, in which he was one of the earliest workers. In 1840 a paper on the phosphates and arsenates, often regarded as a weaker work, was refused by the Royal Society, and he was so incensed that he published it himself. He took the same course soon afterwards with four other papers, two of which On the quantity of acids, bases and salts in different varieties of salts and On a new and easy method of analysing sugar, contain his discovery, regarded by him as second in importance only to the atomic theory, that certain anhydrates, when dissolved in water, cause no increase in its volume, his inference being that the salt enters into the pores of the water.
Dalton's experimental method
As an investigator, Dalton was content with rough and inaccurate instruments, though better ones were readily attainable. Sir Humphry Davy described him as a very coarse experimenter, who almost always found the results he required, trusting to his head rather than his hands.
In the preface to the second part of vol. i. of his New System he says he had so often been misled by taking for granted the results of others that he determined to write as little as possible but what I can attest by my own experience, but this independence he carried so far that it sometimes resembled lack of receptivity. Thus he distrusted, and probably never fully accepted, Gay-Lussac's conclusions as to the combining volumes of gases. He held peculiar and quite unfounded views about chlorine. Even after its elementary character had been settled by Davy, he persisted in using the atomic weights he himself had adopted, even when they had been superseded by the more accurate determinations of other chemists. He always objected to the chemical notation devised by Jöns Jakob Berzelius, although by common consent it was much simpler and more convenient than his own cumbersome system of circular symbols. His library, he was once heard to declare, he could carry on his back, yet reputedly he had not read half the books it contained.
Public life
Before he had propounded the atomic theory he had already attained a considerable scientific reputation. In 1804 he was chosen to give a course of lectures on natural philosophy at the Royal Institution in London, where he delivered another course in 1809–1810. However, he was deficient, it would seem, in the qualities that make an attractive lecturer, being harsh and indistinct in voice, ineffective in the treatment of his subject, and singularly wanting in the language and power of illustration.
In 1810 he was asked by Davy to offer himself as a candidate for the fellowship of the Royal Society, but declined, possibly for financial reasons. However, in 1822 he was proposed without his knowledge, and on election paid the usual fee. Six years previously he had been made a corresponding member of the French Académie des Sciences, and in 1830 he was elected as one of its eight foreign associates in place of Davy.
In 1833 Lord Grey's government conferred on him a pension of £150, raised in 1836 to £300.
Dalton never married, though there is evidence that he enjoyed the company of educated and refined women. He lived for more than a quarter of a century with his friend the Rev. W. Johns (1771–1845), in George Street, Manchester, where his daily round of laboratory work and tuition was broken only by annual excursions to the Lake District and occasional visits to London. In 1822 he paid a short visit to Paris , where he met many distinguished resident scientists. He attended several of the earlier meetings of the British Association at York, Oxford, Dublin and Bristol.
Death and legacy
Dalton died in Manchester in 1844 of paralysis. He had suffered a first attack in 1837, and a second in 1838 had left him enfeebled, both physically and mentally, though he remained able to make experiments. In May 1844 he had another stroke and on July 26 he recorded with trembling hand his last meteorological observation. On the 27th he fell from his bed and was found lifeless by his attendant.
A bust of him, by Francis Legatt Chantrey, was publicly subscribed for him and placed in the entrance hall of the Royal Manchester Institution. It now stands in the entrance to Manchester Town Hall.
Dalton had requested that his eyes be examined after his death, in an attempt to discover the cause of his colour-blindness. He had hypothesised that his aqueous humour might be coloured blue. Post-mortem examination showed that the humours of the eye were perfectly normal. However, an eye was preserved at the Royal Institution, and a 1990s study on DNA extracted from the eye showed that he had lacked the pigment that gives sensitivity to the colour green, the classic condition known as a deuteranope.
In honour of his work with ratios and chemicals that led to the idea of atoms and atomic weights, many chemists and biochemists use the (still unofficial) unit dalton (abbreviated Da) to denote one atomic mass unit, or 1/12 the weight of a neutral atom of carbon-12.
John Dalton's records, carefully preserved for a century, were destroyed during the World War II bombing of Manchester. It is not only the living who are killed in war. - Isaac Asimov.
The road John Dalton Street in the centre of Manchester is named after him, as is John Dalton House at the address 121 Deansgate.
- Roscoe & Harden (1896)
- Laboratory notebooks for 1802–1804, under the date 6th September 1803, on p.248
- Roscoe & Harden (1896), pp. 50,51
Bibliography
- Henry, Life of Dalton, Cavendish Society (1854)
- Angus Smith, Memoir of John Dalton and History of the Atomic Theory
- Roscoe and Harden, A New View of the Origin of Dalton's Atomic Theory (1896)
- DM Hunt, KS Dulai, JK Bowmaker, JD Mollon, "The chemistry of John Dalton's color blindness." Science Feb 17 1995
Physics Encyclopedia
Retrieved from "http://en.wikipedia.org/" All text is available under the terms of the GNU Free Documentation License
Home - Hellenica World
John Dalton's Atomic Theory
- Famous Chemists
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
You may take it for granted that matter is made up of atoms , but what we consider common knowledge was unknown until relatively recently in human history. Most science historians credit John Dalton , a British physicist, chemist, and meteorologist, with the development of modern atomic theory.
Early Theories
While the ancient Greeks believed atoms made matter, they disagreed on what atoms were. Democritus recorded that Leucippus believed atoms to be small, indestructible bodies that could combine to change properties of matter. Aristotle believed elements each had their own special "essence," but he did not think the properties extended down to tiny, invisible particles. No one really questioned Aristotle's theory, since tools did not exist to examine matter in detail.
Along Comes Dalton
So, it wasn't until the 19th century that scientists conducted experiments on the nature of matter. Dalton's experiments focused on gases -- their properties, what happened when they were combined, and the similarities and differences between different types of gases. What he learned led him to propose several laws, which are known collectively as Dalton's Atomic Theory or Dalton's Laws:
- Atoms are small, chemically indestructible particles of matter. Elements consist of atoms.
- Atoms of an element share common properties.
- Atoms of different elements have different properties and different atomic weights.
- Atoms that interact with each other obey the Law of Conservation of Mass . Essentially, this law states the number and kinds of atoms that react are equal to the number and kinds of atoms in the products of a chemical reaction.
- Atoms that combine with each other obey the Law of Multiple Proportions . In other words, when elements combine, the ratio in which the atoms combine can be expressed as a ratio of whole numbers.
Dalton is also known for proposing gas laws ( Dalton's Law of Partial Pressures ) and explaining color blindness. Not all of his scientific experiments could be called successful. For example, some believe the stroke he suffered might have resulted from research using himself as a subject, in which he poked himself in the ear with a sharp stick to “investigate the humours that move inside of my cranium.”
- Grossman, M. I. (2014). "John Dalton and the London atomists: William and Bryan Higgins, William Austin, and new Daltonian doubts about the origin of the atomic theory." Notes and Records . 68 (4): 339–356. doi: 10.1098/rsnr.2014.0025
- Levere, Trevor (2001). Transforming Matter: A History of Chemistry from Alchemy to the Buckyball . Baltimore, Maryland: The Johns Hopkins University Press. pp. 84–86. ISBN 978-0-8018-6610-4.
- Rocke, Alan J. (2005). "In Search of El Dorado: John Dalton and the Origins of the Atomic Theory." Social Research. 72 (1): 125–158. JSTOR 40972005
- Biography of John Dalton, the 'Father of Chemistry'
- J.J. Thomson Atomic Theory and Biography
- Biography of Ernest Rutherford
- Biography of Amedeo Avogadro, Influential Italian Scientist
- Biography of Dmitri Mendeleev, Inventor of the Periodic Table
- Robert Hooke Biography (1635 - 1703)
- Biography of Pierre Curie, Influential French Physicist, Chemist, Nobel Laureate
- How to Measure Volume and Density
- Women in Chemistry - Famous Female Chemists
- Profiles of Famous Black Scientists
- 11 Black Chemists and Chemical Engineers
- Who Is the Father of Chemistry?
- Nobel Prize in Chemistry
- Famous Scientists Who Contributed to Chemistry
- Famous Scientist Pictures - C Names
- A Brief History of Atomic Theory
Reset password New user? Sign up
Existing user? Log in
Dalton's Atomic Model
Already have an account? Log in here.
- Josh Silverman
- Silas Hundt
Dalton's atomic model sets up the building blocks for others to improve on. Though some of his conclusions were incorrect, his contributions were vital. He defined an atom as the smallest indivisible particle .
John Dalton
Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Here's how he defined the atom:
"Matter, though divisible in an extreme degree, is nevertheless not infinitely divisible. That is, there must be some point beyond which we cannot go in the division of matter. I have chosen the word “atom” to signify these ultimate particles." -John Dalton
Basic Laws of Atomic Theory
Dalton's atomic theory, dalton's model of an atom.
Let's review the three basic laws before we get into Dalton's theory.
1. Law of conservation of mass The law of conservation of mass states that the net change in mass of the reactants and products before and after a chemical reaction is zero. This means mass can neither be created nor destroyed. In other words, the total mass in a chemical reaction remains constant. This law was formulated by Antoine Lavoisier in 1789. It was later found to be slightly inaccurate, as in the course of chemical reactions mass can interconvert with heat and bond energy. However, these losses are very small, several orders of magnitude smaller than the mass of the reactants, so that this law is an excellent approximation.
Does the following chemical reaction obey the law of conservation of mass? \[\ce{Ca(OH)2 + CO2 -> CaCO3 + H2O}\] The mass of \(\ce{Ca}\), \(\ce O\), \(\ce H,\) and \(\ce C\) are 40u, 16u, 1u, and 12u, respectively. Yes, they obey the law of conservation of mass. Let's verify it. The molecular mass of \[\begin{align} \ce{Ca(OH)2}&= 40+32+2\\&=74 \\ \\ \ce{CO2}&=12+32\\&=44 \\ \\ \ce{CaCO3}&=40+12+48\\&=100\\ \\ \ce{H2O}&=2+16\\&=18. \end{align}\] Substituting these values in the equation, \[\begin{align}74+44 & = 100+18\\118 & =118.\ _\square \end{align}\]
Does the following chemical reaction obey the law of conservation of mass?
\[\ce{Fe + H_2SO_4 -> FeSO_4 + H_2}\]
\(\) Take \(\text{Fe=55u, H=1u, S=32u, O=16u}\).
2. Law of constant proportions The law of constant proportions states that when a compound is broken, the masses of the constituent elements remain in the same proportion. Or, in a chemical compound, the elements are always present in definite proportions by mass. It means each compound has the same elements in the same proportions, irrespective of where the compound was obtained, who prepared the compound, or the mass of the compound. This law was formulated and proven by Joseph Louis Proust in 1799.
When 1.375 g of cupric oxide is reduced on heating in a current of hydrogen, the weight of copper remaining is 1.098 g. In another experiment, 1.179 g of copper is dissolved in nitric acid and the resulting copper nitrate is converted into cupric oxide by ignition. The weight of cupric oxide formed is 1.476 g.
Does this situation verify law of constant proportions?
A person living in Australia sent a \(100\text{ ml}\) sample of \(\ce{CaCO3}\)(calcium carbonate) to a person living in India. The person living in India made his own sample of \(200\text{ ml}\) and compared it to his friend's. Which of the two compounds has a greater ratio of \(\ce{Ca}:\ce C?\) Both contain equal ratio of \(\ce{Ca}\) and \(\ce C\). This is guaranteed by the law of constant proportions. \(_\square\)
3. Law of multiple proportions The law of multiple proportions states that when two elements form two or more compounds between them, the ratio of the masses of the second element in each compound can be expressed in the form of small whole numbers. This law was proposed by John Dalton, and it is a combination of the previous laws.
Carbon combines with oxygen to form two different compounds (under different circumstances); one is the most common gas \(\ce{CO2}\) and the other is \(\ce{CO}\). Do they obey the law of multiple proportions? Yes, they do obey the law of multiple proportions. Let's verify it. We know that the mass of carbon is \(12\text{ u}\) and that of oxygen is \(16\text{ u}\). So, we can say that \(12\text{ g}\) of carbon combines with \(32\text{ g}\) of oxygen to form \(\ce{CO2}\). Similarly, \(12\text{ g}\) of carbon combines with \(16\text{ g}\) of oxygen to form \(\ce{CO}\). So, the ratio of oxygen in the first and second compound is \(\frac{32}{16}=\frac21=2,\) which is a whole number. \(_\square\)
Two different compounds are formed by the elements carbon and oxygen. The first compound contains 42.9% by mass carbon and 57.1% by mass oxygen. The second compound contains 27.3% by mass carbon and 72.7% by mass oxygen.
Does this obey the law of multiple proportions?
There is one other law which was proposed to find the relation between two different compounds.
4. Law of reciprocal proportions The law of reciprocal proportions states that when two different elements combine with the same quantity of a third element, the ratio in which they do so will be the same or a multiple of the proportion in which they combine with each other. This law was proposed by Jeremias Ritcher in 1792.
Dalton picked up the idea of divisibility of matter to explain the nature of atoms. He studied the laws of chemical combinations (the laws we discussed in the previous section) carefully and came to a conclusion about the characteristics of atoms.
His statements were based on the three laws we'd discussed earlier. He stated the following postulates (not all of them are true) about his atomic theory.
- Matter is made of very tiny particles called atoms .
- Atoms are indivisible structures , which can neither be created nor destroyed during a chemical reaction (based on the law of conservation of mass).
- All atoms of a particular element are similar in all respects , be it their physical or chemical properties.
- Inversely, atoms of different elements show different properties , and they have different masses and different chemical properties.
- Atoms combine in the ratio of small whole numbers to form stable compounds, which is how they exist in nature.
- The relative number and the kinds of atoms in a given compound are always in a fixed ratio (based on the law of constant proportions).
As said earlier, all the postulates weren't correct. Let us discuss the drawbacks of Dalton's atomic theory.
- The first part of the second postulate was not accepted. Bohr's model proposed that the atoms could be further divided into protons, neutrons, and electrons.
- The third postulate was also proven to be wrong because of the existence of isotopes , which are atoms of the same element but of different masses.
- The fourth postulate was also proven to be wrong because of the existence of isobars , which are atoms of different elements but of the same mass.
Nonetheless, to propose the idea of an atom (considering the time period) is a great achievement, and we must appreciate Dalton's work.
Based on all his observations, Dalton proposed his model of an atom. It is often referred to as the billiard ball model . He defined an atom to be a ball-like structure, as the concepts of atomic nucleus and electrons were unknown at the time. If you asked Dalton to draw the diagram of an atom, he would've simply drawn a circle!
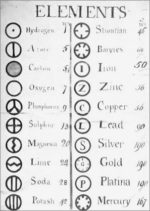
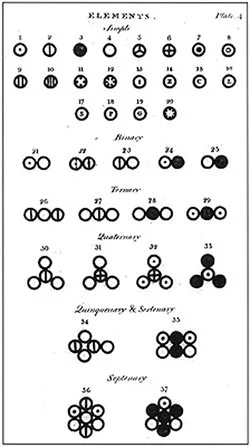
Later, he tried to symbolize atoms, and he became one of the first scientists to assign such symbols. He gave a specific symbol to each atom (see below).
It was only after J. J. Thompson proposed his model that the true concepts had come into existence. Later, Rutherford worked on Dalton's and Thompson's models and brought out a roughly correct shape of the concept. Finally, Bohr's model and the quantum mechanical model gave a complete model which we know of today.
Atoms, Molecules, Elements, Compounds
Bohr's Model
Problem Loading...
Note Loading...
Set Loading...
We’re fighting to restore access to 500,000+ books in court this week. Join us!
Internet Archive Audio

- This Just In
- Grateful Dead
- Old Time Radio
- 78 RPMs and Cylinder Recordings
- Audio Books & Poetry
- Computers, Technology and Science
- Music, Arts & Culture
- News & Public Affairs
- Spirituality & Religion
- Radio News Archive

- Flickr Commons
- Occupy Wall Street Flickr
- NASA Images
- Solar System Collection
- Ames Research Center

- All Software
- Old School Emulation
- MS-DOS Games
- Historical Software
- Classic PC Games
- Software Library
- Kodi Archive and Support File
- Vintage Software
- CD-ROM Software
- CD-ROM Software Library
- Software Sites
- Tucows Software Library
- Shareware CD-ROMs
- Software Capsules Compilation
- CD-ROM Images
- ZX Spectrum
- DOOM Level CD

- Smithsonian Libraries
- FEDLINK (US)
- Lincoln Collection
- American Libraries
- Canadian Libraries
- Universal Library
- Project Gutenberg
- Children's Library
- Biodiversity Heritage Library
- Books by Language
- Additional Collections

- Prelinger Archives
- Democracy Now!
- Occupy Wall Street
- TV NSA Clip Library
- Animation & Cartoons
- Arts & Music
- Computers & Technology
- Cultural & Academic Films
- Ephemeral Films
- Sports Videos
- Videogame Videos
- Youth Media
Search the history of over 866 billion web pages on the Internet.
Mobile Apps
- Wayback Machine (iOS)
- Wayback Machine (Android)
Browser Extensions
Archive-it subscription.
- Explore the Collections
- Build Collections
Save Page Now
Capture a web page as it appears now for use as a trusted citation in the future.
Please enter a valid web address
- Donate Donate icon An illustration of a heart shape
John Dalton and the atomic theory; the biography of a natural philosopher
Bookreader item preview, share or embed this item, flag this item for.
- Graphic Violence
- Explicit Sexual Content
- Hate Speech
- Misinformation/Disinformation
- Marketing/Phishing/Advertising
- Misleading/Inaccurate/Missing Metadata
![[WorldCat (this item)] [WorldCat (this item)]](https://archive.org/images/worldcat-small.png)
plus-circle Add Review comment Reviews
155 Previews
4 Favorites
DOWNLOAD OPTIONS
No suitable files to display here.
EPUB and PDF access not available for this item.
IN COLLECTIONS
Uploaded by station07.cebu on February 19, 2020
SIMILAR ITEMS (based on metadata)
Dalton, John
- Reference work entry
- First Online: 01 January 2023
- Cite this reference work entry

- Stephen Westland 2

This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Dickinson, C., Murray, I., Carden, D.: Dalton – letter. In: Dickinson, C., Murray, I., Carden, D. (eds.) John Dalton’s Colour Vision Legacy. Taylor & Francis, London (1997)
Google Scholar
Mollon, J.D., Dulai, K.S., Hunt, D.M.: Dalton’s colour blindness: an essay in molecular biography. In: Mollon, J.D., Dulai, K.S., Hunt, D.M., Dickinson, C., Murray, I., Carden, D. (eds.) John Dalton’s Colour Vision Legacy. Taylor & Francis, London (1997)
Nash, L.K.: The origin of Dalton’s chemical atomic theory. Isis. 47 (2), 101–116 (1956) The History of Science Society CrossRef
Article Google Scholar
Download references
Author information
Authors and affiliations.
Colour Science and Technology, University of Leeds, Leeds, UK
Stephen Westland
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stephen Westland .
Editor information
Editors and affiliations.
North Carolina State University, Color Science & Imaging Laboratories, Raleigh, NC, USA
Renzo Shamey
Section Editor information
School of Design, University of Leeds, Leeds, UK
Ming Ronnier Luo
University of Zhejiang, Zhejiang, China
National Taiwan University of Science and Technology, Taipei City, Taiwan
Rights and permissions
Reprints and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry.
Westland, S. (2023). Dalton, John. In: Shamey, R. (eds) Encyclopedia of Color Science and Technology. Springer, Cham. https://doi.org/10.1007/978-3-030-89862-5_338
Download citation
DOI : https://doi.org/10.1007/978-3-030-89862-5_338
Published : 28 September 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-89861-8
Online ISBN : 978-3-030-89862-5
eBook Packages : Physics and Astronomy Reference Module Physical and Materials Science Reference Module Chemistry, Materials and Physics
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 03 September 1966
John Dalton the Atomist
- J. ROSE 1
Nature volume 211 , pages 1015–1016 ( 1966 ) Cite this article
54 Accesses
Metrics details
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Lavoisier, A. L., Traité de Chimie , i, 31 (1789).
Partington, J. R., A History of Chemistry , 3 , 771 (Macmillan, London, 1962).
Book Google Scholar
Dalton, J., A New System of Chemical Philosophy , Part 1, 187 (S. Russell (for R. Bickerstaff), London and Manchester, 1808).
Google Scholar
Dalton, J. (ref. 3), 70–71.
Partington, J. R. (ref. 2), 778–782.
Partington, J. R., Scientia , 49 , 221 (1955).
Higgins, W., Comparative View , 46, 48, 192 and 262 (J. Murray, London, 1789).
Meldrum, A. N., Chemical News , 102 , 1 (1910).
CAS Google Scholar
Greenaway, F., Endeavour , 25 , 73 (1966).
Article CAS Google Scholar
Nash, L. K., Isis , 47 , 101 (1956).
Dalton, J. (ref. 3), 213.
Dalton, J., A New System of Chemical Philosophy , Part 1, 2 , 351 (G. Wilson, London, 1827).
Download references
Author information
Authors and affiliations.
Blackburn College of Technology and Design,
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
About this article
Cite this article.
ROSE, J. John Dalton the Atomist. Nature 211 , 1015–1016 (1966). https://doi.org/10.1038/2111015a0
Download citation
Issue Date : 03 September 1966
DOI : https://doi.org/10.1038/2111015a0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Dalton Transactions
John dalton – the man and the myth.

a University of Basel, Department of Chemistry, BPR 1096, Mattenstrasse 24a, CH-4058 Basel, Switzerland E-mail: [email protected] Tel: +41 61 207 1001
John Dalton is one of the pioneers who transformed chemistry into the science that we enjoy today. His name is irrevocably linked with the atomic theory that underlies our modern understanding of chemical structure. This article summarizes his life and contributions and attempts to place them in the context of the intellectual revolution that was transforming all aspects of science.
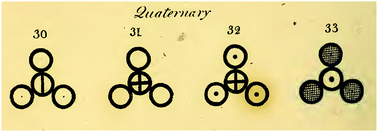
- This article is part of the themed collections: Dalton Transactions up-and-coming articles , 2022 Frontier and Perspective articles and Dalton turns 50 – celebrating our board members past and present
Article information
Download Citation
Permissions.
E. C. Constable, Dalton Trans. , 2022, 51 , 768 DOI: 10.1039/D1DT04135E
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence . You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author, advertisements.

IMAGES
VIDEO
COMMENTS
John Dalton (born September 5 or 6, 1766, Eaglesfield, Cumberland, England—died July 27, 1844, Manchester) was an English meteorologist and chemist, a pioneer in the development of modern atomic theory.. Early life and education. Dalton was born into a Quaker family of tradesmen; his grandfather Jonathan Dalton was a shoemaker, and his father, Joseph, was a weaver.
Birth date: September 6, 1766. Birth City: Eaglesfield. Birth Country: United Kingdom. Gender: Male. Best Known For: Chemist John Dalton is credited with pioneering modern atomic theory. He was ...
John Dalton was born on September 6, 1766, in Eaglesfield, a small village in Cumberland, England. He was the third of six children born to Joseph Dalton, a weaver, and Deborah Greenup. The Dalton family belonged to the Quaker faith, which emphasized values such as simplicity, hard work, and education. These principles shaped John Dalton's ...
John Dalton (September 6, 1766-July 27, 1844) was a renowned English chemist, physicist, and meteorologist. His most famous contributions were his atomic theory and color blindness research. Parents: Joseph Dalton, Deborah Greenups. Notable Quote: "Matter, though divisible in an extreme degree, is nevertheless not infinitely divisible.
Engraving of John Dalton by William Henry Worthington after a painting by William Allen, 1823. Science History Institute. In explaining the law of partial pressures to skeptical chemists of the day—including Humphry Davy —Dalton claimed that the forces of repulsion thought to cause pressure acted only between atoms of the same kind and that ...
John Dalton FRS (/ ˈ d ɔː l t ən /; 5 or 6 September 1766 - 27 July 1844) was an English chemist, physicist and meteorologist. [1] He introduced the atomic theory into chemistry. He also researched colour blindness ; as a result, the umbrella term for red-green congenital colour blindness disorders is Daltonism in several languages.
Biography of John Dalton John Dalton, an English chemist and physicist, is known for his contributions to the development of atomic theory. He established the law of multiple proportions in 1803 and introduced the concept of atomic weight. Dalton was the first to determine the atomic weights of several elements and discovered the gas laws that were named after him.
John Dalton Biography. John Dalton (6 September 1766 - 27 July 1844) was an English Chemist who introduced atomic theory into chemistry, revolutionising the subject and laying the foundations for modern chemistry as we understand it. Dalton proposed ways to measure atomic weight and strengthened the importance of atoms in chemistry.
Died. July 27, 1844 (at age 77) Manchester, England. Nationality. English. John Dalton was an English scientist who was born in Eaglesfield, near Cockermouth, in the northwest region of England in 1766. Despite the fact that he was born into a poor family of weavers, Dalton embraced education and proved to be a very precocious child.
Erik Gregersen. John Dalton - Atomic Theory, Chemistry, Physics: By far Dalton's most influential work in chemistry was his atomic theory. Attempts to trace precisely how Dalton developed this theory have proved futile; even Dalton's own recollections on the subject are incomplete. He based his theory of partial pressures on the idea that ...
John Dalton, (born Sept. 5 or 6, 1766, Eaglesfield, Cumberland, Eng.—died July 27, 1844, Manchester), British chemist and physicist.He spent most of his life in private teaching and research. His work on gases led him to state Dalton's law (see gas laws). He devised a system of chemical symbols, ascertained the relative weights of atoms, and arranged them into a table.
Cite this: Dalton Trans., 2022, 51, 768 Received 8th December 2021, Accepted 23rd December 2021 DOI: 10.1039/d1dt04135e rsc.li/dalton. John Dalton - the man and the myth. Edwin C. Constable. John Dalton is one of the pioneers who transformed chemistry into the science that we enjoy today. His name is irrevocably linked with the atomic theory ...
Born in September 1766, John Dalton was an English scientist who did pioneering work in the fields of chemistry and meteorology. He was the first to publish a paper on colour blindness and also provided great new insights into the nature of gases. He was renowned during his life though the enormous nature of his contribution was realized with further advancements in science.
John Dalton (September 6, 1766 July 27, 1844) was a British chemist and physicist, born at Eaglesfield, near Cockermouth in Cumberland.He is most well known for his advocacy of the atomic theory. Biography. Early life. Dalton received his early education from his father and from John Fletcher, a teacher of the Quaker school at Cumberland, on whose retirement in 1778 he himself started teaching.
This blog delves into the life, work, and legacy of John Dalton, exploring his contributions in a detailed and structured manner. John Dalton Early Life and Education. John Dalton was born on September 6, 1766, in the small village of Eaglesfield in Cumberland, England. He came from a modest Quaker family, with his father working as a weaver.
What he learned led him to propose several laws, which are known collectively as Dalton's Atomic Theory or Dalton's Laws: Atoms are small, chemically indestructible particles of matter. Elements consist of atoms. Atoms of an element share common properties. Atoms of different elements have different properties and different atomic weights.
Dalton's atomic model sets up the building blocks for others to improve on. Though some of his conclusions were incorrect, his contributions were vital. He defined an atom as the smallest indivisible particle. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Here's how he defined ...
John Dalton and the atomic theory; the biography of a natural philosopher ... "'John Dalton was a very singular man. A Quaker by profession and practice, he had none of the manners or ways of the world. A tolerable mathematician, he gained his livelihood, I believe, by teaching mathematics to young people ... ; he followed with ardour analogies ...
Thirty-six years prior to Dalton's famous 1794 letter, Thomas Young had postulated that congenital color vision defects arose from the photoreceptors, but Dalton refused to believe it [].As a result of his meticulous observations, Dalton believed that the fluids in his eyes must contain a blue colorant, and he instructed that on his death his eyes be subject to a postmortem analysis.
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
By DR. J. ROSE. JOHN DALTON was born at Eaglcsfield in Cumberland on September 6, 1766, of Quaker parentage. He himself remained a Quaker all his life, and his teachers, and friends during his ...
John Dalton is one of the pioneers who transformed chemistry into the science that we enjoy today. His name is irrevocably linked with the atomic theory that underlies our modern understanding of chemical structure. This article summarizes his life and contributions and attempts to place them in the context Dalton Transactions up-and-coming articles 2022 Frontier and Perspective articles ...