

The Biology Corner
Biology Teaching Resources

Observe Photosynthesis with this Easy Experiment

Photosynthesis is a complicated topic that requires students to develop mental models of the phenomenon. Teachers may struggle to find hands-on activities that can be completed in a short class period.
There are several ways this can be accomplished. Students can observe the products created by photosynthesis, oxygen. In this lab, students cut small disks from spinach leaves and record the time it takes to float.
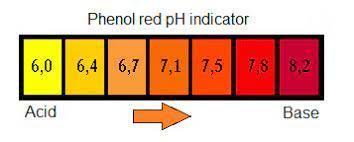
You can also indirectly measure photosynthesis by the amount of carbon dioxide absorbed during the process. I have another lab that can be a demonstration for the whole class where students can see the effects of photosynthesis. In this demonstration, an indicator ( phenol red) is used to measure the amount of carbon taken up during the process.
Class Demonstration
The demonstration is simple. You take leaves from a plant and place it in the indicator solution. The plants exposed to light will consume the carbon dioxide which decreases the acidity of the solution. The yellow solution will turn red!
I have used elodea leaves in the past, but find that these specimens have become hard to acquire. Many states consider this water weed an invasive species and limit shipping. Luckily, leaves from kale will also work. Kale is a fairly inexpensive vegetable you can get at the supermarket.
The demonstration is simple. Phenol red starts out as a red color but will change to yellow if you blow into it with a straw. The carbon dioxide in your breath will change the solution to a yellow color.
Next, place leaves from kale into test tubes with the yellow solution. Place one tube in the light and another in the dark (using aluminum foil).
A full spectrum grow light will have the best results. Leave one test tube empty as a control. In about 20-30 minutes, the test tube in the light will change to red, indicating that the carbon dioxide has been consumed.
You can use this demonstration as an introduction to photosynthesis, or a plant unit. Follow up with a discussion that asks students the following questions:
- Why does phenol red change color when I add carbon dioxide?
- Why does it turn back to red in the tube with a plant exposed to light?
- Why did the plant in the dark not change color?
If you want a more student-directed activity, students can do the lab for themselves. This handout outlines the procedure and includes discussion questions for students to answer in groups.
As an extension activity, ask students what will happen to the tubes if left to sit overnight. Generally, the color will change to yellow as plants respire and release carbon dioxide. (Note: I have had mixed results with this, but it’s a good way to prompt discussion about the relationship between respiration and photosynthesis.)
Shannan Muskopf
- Science, Tech, Math ›
- Chemistry ›
- Projects & Experiments ›
Floating Spinach Disks Photosynthesis Demonstration
Watch Leaves Perform Photosynthesis
Kevan/Flickr/CC BY 2.0
- Projects & Experiments
- Chemical Laws
- Periodic Table
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Watch spinach leaf disks rise and fall in a baking soda solution in response to photosynthesis . The leaf disks intake carbon dioxide from a baking soda solution and sink to the bottom of a cup of water. When exposed to light, the disks use carbon dioxide and water to produce oxygen and glucose. Oxygen released from the leaves forms tiny bubbles that cause the leaves to float.
Photosynthesis Demonstration Materials
You can use other leaves for this project besides spinach. Ivy leaves or pokeweed or any smooth-leaf plant work. Avoid fuzzy leaves or areas of leaves that have large veins.
- fresh spinach leaves
- single hole punch or a hard plastic straw
- baking soda (sodium bicarbonate)
- liquid dishwashing detergent
- plastic syringe (no needle, 10 ccs or larger)
- clear cup or glass
- light source (bright sunlight works or you can use an artificial light)
- Prepare a bicarbonate solution by mixing 6.3 grams (about 1/8 teaspoon) baking soda in 300 milliliters of water. The bicarbonate solution acts as a source of dissolved carbon dioxide for photosynthesis.
- In a separate container, dilute a detergent solution by stirring a drop of dishwashing liquid in about 200 milliliters of water.
- Fill a cup partly full with the baking soda solution. Add a drop of the detergent solution to this cup. If the solution forms suds, add more baking soda solution until you stop seeing bubbles.
- Use the hole punch or straw to punch ten to 20 disks from your leaves. Avoid the edges of the leaves or major veins. You want smooth, flat disks.
- Remove the plunger from the syringe and add the leaf disks.
- Replace the plunger and slowly depress it to expel as much air as you can without crushing the leaves.
- Dip the syringe in the baking soda/ detergent solution and draw in about 3 ccs of liquid. Tap the syringe to suspend the leaves in the solution.
- Push the plunger to expel excess air, then place your finger over the end of the syringe and pull back on the plunger to create a vacuum.
- While maintaining the vacuum, swirl the leaf disks in the syringe. After 10 seconds, remove your finger (release the vacuum).
- You may wish to repeat the vacuum procedure two to three more times to ensure the leaves take up carbon dioxide from the baking soda solution. The disks should sink to the bottom of the syringe when they are ready for the demonstration. If the disks do not sink, use fresh disks and a solution with a higher concentration of baking soda and a bit more detergent.
- Pour the spinach leaf discs into the cup of baking soda/detergent solution. Dislodge any disks that stick to the side of the container. Initially, the disks should sink to the bottom of the cup.
- Expose the cup to light. As the leaves produce oxygen , bubbles forming on the surface of the disks will cause them to rise. If you remove the light source from the cup, the leaves eventually will sink.
- If you return the disks to the light, what happens? You can experiment with the intensity and duration of the light and its wavelength. If you would like to set up a control cup, for comparison, prepare a cup containing water with diluted detergent and spinach leaf disks that have not been infiltrated with carbon dioxide.
- Hydrogen Balloon Explosion Experiment
- Top Halloween Chemistry Projects
- How to Do Paper Chromatography With Leaves
- 10 Cool Chemistry Demonstrations for Educators
- How to Do the Color Change Chameleon Chemistry Demonstration
- How to Do the Barking Dog Chemistry Demonstration
- Leidenfrost Effect Demonstrations
- Elephant Toothpaste Chemistry Demonstration
- How to Perform the Nitrogen Triiodide Chemistry Demonstration
- Water - Wine - Milk - Beer Chemistry Demonstration
- Color Change Chemistry Experiments
- 5 Types of Science Fair Projects
- Create a Magic Genie in a Bottle Effect (Chemistry)
- Sulfuric Acid and Sugar Demonstration
- Interesting High School Chemistry Demonstrations
- How to Perform the Dancing Gummi Bear Demonstration
Explore the natural world through science and sustainability

How to Visualize Photosynthesis: A Simple Science Experiment
Looking for a science experiment that visualizes how photosynthesis works? Check out this simple outdoor science project that requires very few materials and can be done at home or school!

Introducing kids to the process of photosynthesis can be tricky, as this complex chemical process isn’t easily seen; it just kind of “happens” all around us, all the time. The idea that a living organism takes one type of gas from the atmosphere and, with the help of water and sunlight, makes an entirely new type of gas that is essential to our survival, along with food for itself seems almost magical. When looking for a way to help my kids understand how plants perform photosynthesis, I stumbled upon an easy and free experiment that allows them to see the oxygen gas created by green plants.
What is Photosynthesis?
Photosynthesis is the process by which green plants, algae, and some bacteria convert light energy from the sun into chemical energy in the form of glucose (sugar). It is a complex series of chemical reactions that take place in specialized organelles called chloroplasts, which contain the pigment chlorophyll.
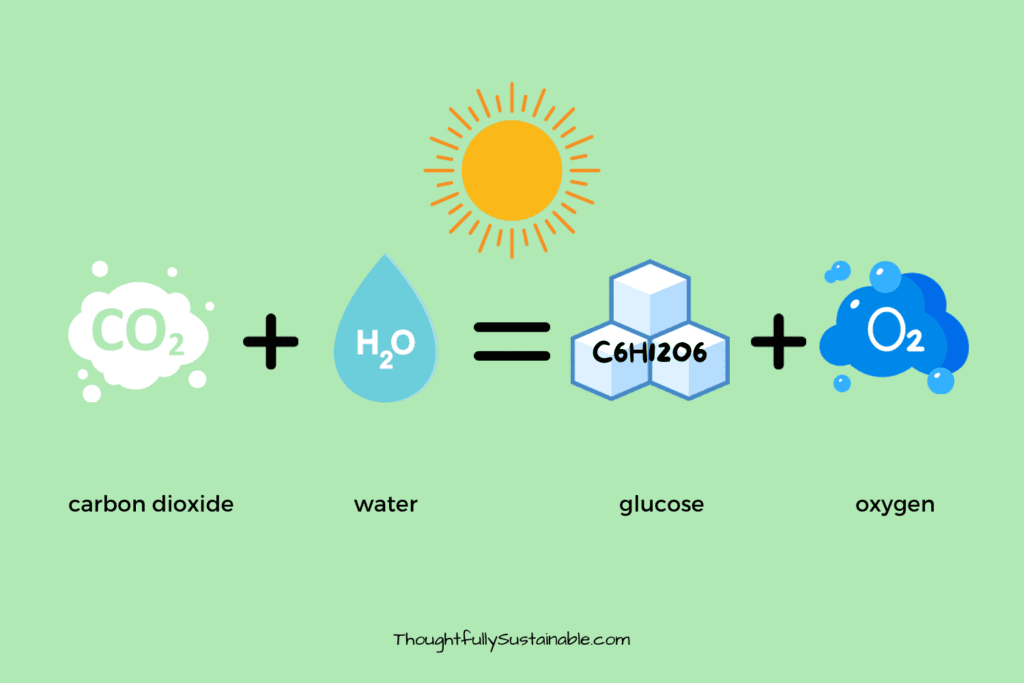
During photosynthesis, carbon dioxide (CO 2 ) from the atmosphere and water (H 2 O) from the soil is combined with light energy to produce glucose and oxygen (O 2 ). This process is essential for life on Earth, as it provides the basis for the food chain and produces the oxygen we breathe.
What is the Chemical Equation for Photosynthesis?
The overall balanced chemical equation for photosynthesis is:
6 CO 2 + 6 H 2 O + light energy → C 6 H 12 O 6 + 6 O 2
where CO 2 is carbon dioxide, H 2 O is water, C 6 H 12 O 6 is glucose, and O 2 is oxygen.
The coefficients in front of each chemical formula give us a bit more information about the “recipe” needed to produce food for the photosynthesizing organism. It takes 6 molecules of carbon dioxide combined with 6 molecules of water in the presence of sunlight to create 1 molecule of glucose and 6 molecules of oxygen.
How Does Photosynthesis Work?
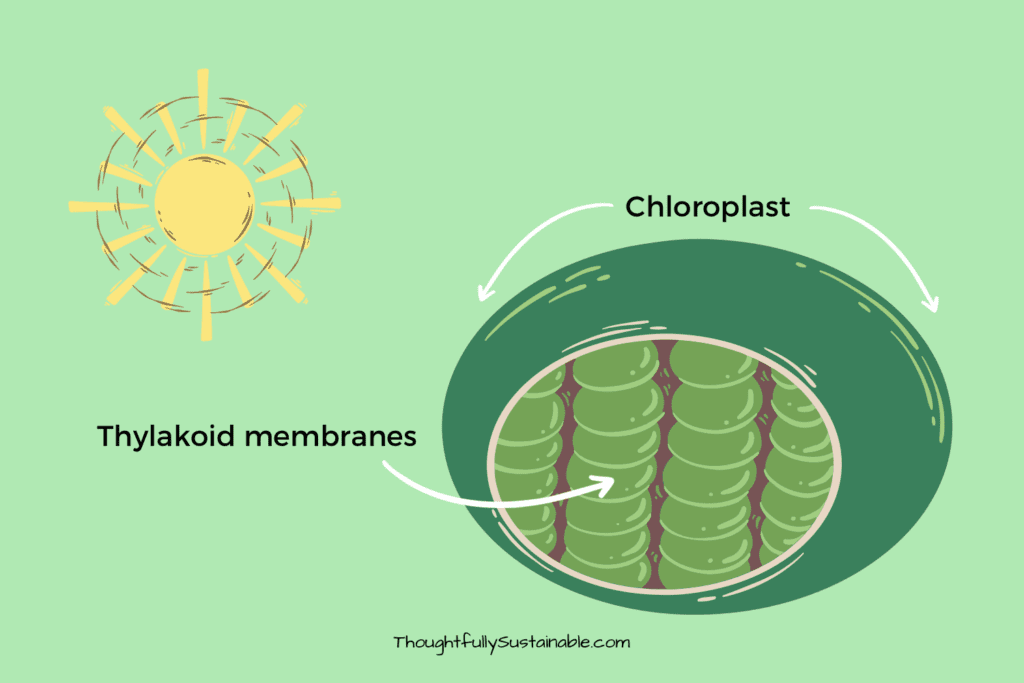
As in most things in science, the process of photosynthesis can be described in even more detail than the general balanced equation shown above. Photosynthesis is a complex process that occurs in two main stages: light-dependent reactions and light-independent reactions (also known as the Calvin cycle). Typically this level of detail wouldn’t be taught to students until high school level biology. Here’s a brief overview of how photosynthesis works in more detail:
Light-dependent reactions:
The first stage of photosynthesis occurs in the thylakoid membrane of the chloroplasts, where light energy is absorbed by chlorophyll and other pigments. This light energy is used to create high-energy molecules such as ATP (adenosine triphosphate) and NADPH (nicotinamide adenine dinucleotide phosphate), which are needed for the second stage of photosynthesis.
Light-independent reactions (Calvin cycle):
The second stage of photosynthesis occurs in the stroma of the chloroplasts, where carbon dioxide is fixed into organic molecules such as glucose. This process is known as the Calvin cycle and requires the ATP and NADPH produced in the first stage. In the Calvin cycle, carbon dioxide molecules are combined with molecules of the 5-carbon sugar ribulose bisphosphate (RuBP) to form an unstable 6-carbon molecule. This molecule is then broken down into two molecules of a 3-carbon sugar, which can be used to create glucose and other organic molecules.
Overall, photosynthesis is a complex biochemical process that converts light energy into chemical energy, producing oxygen and organic molecules as byproducts.

What Types of Organisms Use Photosynthesis?
Photosynthesis is used by a wide range of organisms to produce food. The most well-known photosynthetic organisms are green plants, which use chlorophyll to convert light energy into glucose. However, many other types of organisms use photosynthesis as a source of food, including:
- Algae: These are a diverse group of photosynthetic organisms that can be found in a wide range of environments, including freshwater, marine, and terrestrial habitats. They come in a variety of shapes and sizes, ranging from single-celled organisms to large, multicellular seaweeds.
- Cyanobacteria: These are a group of photosynthetic bacteria that are capable of fixing nitrogen from the atmosphere. They are often found in aquatic environments, but can also be found in soil, on rocks, and in other habitats.
- Photosynthetic bacteria: In addition to cyanobacteria, other types of bacteria are capable of photosynthesis, including purple bacteria and green sulfur bacteria.
For our purposes of creating a simple photosynthesis science experiment, we will be focusing on green plants.

Free Visualizing Photosynthesis Science Experiment Printable
To help make this science experiment more meaningful, I’ve created a free printable that you can use to guide your students’ learning. The printable includes:
- Creation of a hypothesis
- Visual observations before and after the experiment
- Analysis and conclusion questions
To get your copy of this free printable, simply enter your name and email address below!
FREE Visualizing Photosynthesis Science Experiment
Materials needed for visualizing photosynthesis science experiment.
This simple science experiment requires very few materials and can be set up within minutes. Here are the supplies needed to conduct this visualizing photosynthesis science experiment.
- 5-7 freshly picked green leaves
- 5-7 small pebbles or other small objects to weigh down the leaves
- shallow dish or tray with sides
- direct sunlight
- free printable “Visualizing Photosynthesis” student sheets
I have found that the results of this experiment are best when it is conducted outside with access to direct sunlight, as opposed to running the experiment inside in front of a window. However, if you have access to a greenhouse, or have old windows that are not double-paned, this experiment may do well indoors.

Instructions to Conduct the Visualizing Photosynthesis Science Experiment
The initial set-up of this science experiment is quite simple and, depending upon the time of year it is conducted, you may begin to see results within an hour.
- On the free printable provided, make a hypothesis about what you think will happen to the surface of the leaves when left undisturbed in direct sunlight for an hour.
- Place 5-7 freshly picked leaves face up in a shallow dish or tray.
- Position the dish in direct sunlight.
- Sketch one or two of the leaves chosen for your experiment.
- Place a small pebble on the center of each leaf. Be careful not to cover the entire leaf with your object, as sunlight needs to be able to reach the leaf.
- Pour enough water into the dish to just cover all of the leaves.
- Allow the leaves to sit undisturbed for an hour in direct sunlight.
- After an hour, observe the leaves.
- Create a second sketch of the leaves you chose at the beginning of the experiment, noting any differences that have occurred. Make sure to take a close look at the surface and edges of the leaves.
- If no changes have occurred, allow the leaves to sit undisturbed for another hour in direct sunlight, then reobserve.
- Answer the questions that are found on the visualizing photosynthesis printable .

Typical Results of the Visualizing Photosynthesis Science Experiment
Once the leaves have sat undisturbed for one hour in direct sunlight, learners should see small bubbles form on the edges and tops of the leaves. These bubbles contain oxygen and are the direct result of photosynthesis. The purpose of the water in the experiment is to visualize the oxygen gas; without the water, the gas bubbles would not be trapped and would simply enter the air.
Be sure to have the students complete the questions on my free printable on their own or in small groups before discussing it as a class. It may be helpful to display the chemical equation for photosynthesis to remind students that the creation of oxygen gas is part of photosynthesis.
More Science Experiments About Plants
If you enjoyed this simple science experiment about plants, you may also like the following:
- How to Propagate Plants in Water with Kids
- How to Regrow Vegetables from Food Scraps
- How to Grow Your Own Popcorn
- Teaching Kids How to Grow Potatoes
How to Visualize Photosynthesis: A Science Experiment

Instructions
To download the free printable for this science experiment, click here .
Similar Posts

Full and Unpaid Review of the OMAX 40X-2500X Digital Lab Trinocular Compound LED Microscope

How Road Salt Effects the Environment: A Science Lesson for Kids
Composting Coloring Page for Kids: A Free Printable
Explore 3D Printing with Kids + Free Printable Activities

Explore the World of Jane Goodall: A Famous Female Scientist

Learning About Eugenie Clark: A Famous Female Scientist
Remember Me

Experiment Photosynthesis and Respiration (CO2 and O2) Experiments
Photosynthesis and respiration (co 2 and o 2 ).
Experiment #31C from Biology with Vernier
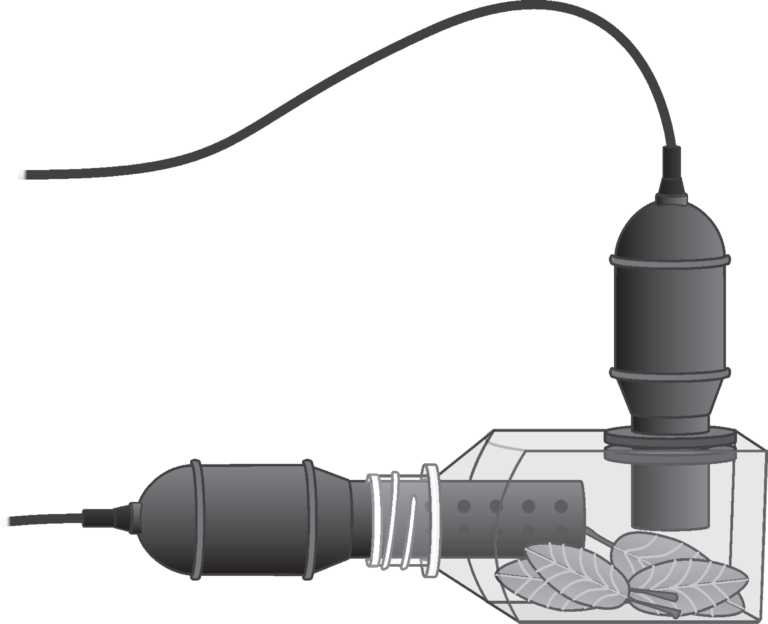
Introduction
Plants make sugar, storing the energy of the sun into chemical energy, by the process of photosynthesis. When they require energy, they can tap the stored energy in sugar by a process called cellular respiration.
The process of photosynthesis involves the use of light energy to convert carbon dioxide and water into sugar, oxygen, and other organic compounds. This process is often summarized by the following reaction:
Cellular respiration refers to the process of converting the chemical energy of organic molecules into a form immediately usable by organisms. Glucose may be oxidized completely if sufficient oxygen is available by the following equation:
All organisms, including plants and animals, oxidize glucose for energy. Often, this energy is used to convert ADP and phosphate into ATP.
In this experiment, you will
- Use an O 2 Gas Sensor to measure the amount of oxygen gas consumed or produced by a plant during respiration and photosynthesis.
- Use a CO 2 Gas Sensor to measure the amount of carbon dioxide consumed or produced by a plant during respiration and photosynthesis.
- Determine the rate of respiration and photosynthesis of a plant.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.

Ready to Experiment?
Ask an expert.
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email [email protected]
Purchase the Lab Book
This experiment is #31C of Biology with Vernier . The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.

How Is Carbon Dioxide Absorbed During Photosynthesis?

Plants use energy from light to convert water and carbon dioxide into sugar and oxygen in a process called photosynthesis. Chlorophyll, the green pigment in leaves, absorbs sunlight and uses the energy to convert six molecules of carbon dioxide and six molecules of water into one molecule of sugar and six molecules of oxygen. Plants use the sugar to grow and release the oxygen back into the atmosphere. They also help regulate the amount of carbon dioxide, which is one of the most important greenhouse gases, in the atmosphere.
Leaf Structure
Plant leaves have small openings, called stomata, all over their surfaces. The stomata open to absorb the carbon dioxide needed to perform photosynthesis. They also open to release the oxygen produced by this process. Plant roots and leaves absorb water, which reacts with carbon dioxide using energy from light as the catalyst. Plant leaves are also able to absorb and release water through the stomata.
Greenhouse Gases
Carbon dioxide is a greenhouse gas. It traps heat in the atmosphere, causing the greenhouse effect that contributes to global warming. According to the U.S. Environmental Protection Agency, U.S. greenhouse gas emissions have been steadily increasing; in 2010, U.S. emissions totaled more than 6 billion metric tons of carbon dioxide equivalent. Carbon dioxide is released into the atmosphere when fossil fuels such as natural gas, coal and fuel oil are burned for energy production. Planting trees and other vegetation can help reduce the amount of carbon dioxide in the atmosphere.
Plants as Carbon "Sinks"
Every year, the earth's forests are able to absorb one-third of the carbon dioxide emitted by burning fossil fuels. Forests act as carbon "sinks" and reduce the amount of carbon dioxide in the air significantly. A study by the United States Forest Service found that tropical forests absorb more carbon than forests in temperate or boreal regions. However, tropical forests are disappearing as developing countries replace them with commercial centers and pasture for livestock grazing.

Deforestation Affects the Atmosphere
One of the harmful side effects of deforestation is an increase in atmospheric carbon. Deforestation increases atmospheric carbon dioxide in two ways. Machines that cut and process logs emit carbon dioxide, and cut trees that remain on the forest floor decompose, which releases more carbon dioxide into the atmosphere. The United Nations, through its Intergovernmental Panel on Climate Changes and UN-REDD — Reducing Emissions from Deforestation and forest Degradation — program, works to discourage deforestation in developing countries. The REDD+ program provides financial incentives for developing countries to reduce deforestation by assigning financial value to forests' carbon-storage capabilities.
- EPA: U.S. Greenhouse Gas Inventory Report
- Science: A Large and Persistent Carbon Sink in the World's Forests
- EPA: Trends in Greenhouse Gas Emissions
- New York Times: As 'Sinks' for Carbon, Forests Are Even Mightier Than Assumed
- Intergovernmental Panel of Climate Change
Cite This Article
Cairoli, Sarah. "How Is Carbon Dioxide Absorbed During Photosynthesis?" sciencing.com , https://www.sciencing.com/carbon-dioxide-absorbed-during-photosynthesis-3196/. 10 April 2018.
Cairoli, Sarah. (2018, April 10). How Is Carbon Dioxide Absorbed During Photosynthesis?. sciencing.com . Retrieved from https://www.sciencing.com/carbon-dioxide-absorbed-during-photosynthesis-3196/
Cairoli, Sarah. How Is Carbon Dioxide Absorbed During Photosynthesis? last modified March 24, 2022. https://www.sciencing.com/carbon-dioxide-absorbed-during-photosynthesis-3196/
Recommended
Photosynthesis Lab
Photosynthesis is one of the most important anabolic chemical reactions that allows life to exist on Earth. With water, light energy from the sun, and carbon dioxide from the air, photosynthetic organisms are able to build simple sugars. Organisms that can make their own food are called autotrophs , and are at the base of the food chain. The basic reaction is:
6 C O 2 + 12 H 2 O + e --> 2 C 6 H 12 O 6 + 6 O 2
carbon dioxide + water + light energy --> glucose + oxygen
Oxygen molecules are colored to show their fate. Oxygen from CO 2 ends up in glucose. Oxygen from water becomes free O 2
Photosynthesis has two stages. Stage 1 requires light. Stage 2 can work in the light or in the dark. The energy accumulated in Stage 1 is used to drive Stage 2.
- The light reaction is used to convert sunlight into chemical energy stored in ATP and another energy storage molecule called NADP .
- The light-independent reaction or Calvin Cycle takes carbon dioxide and fixes it in three-carbon molecules which will eventually be synthesized into glucose.
Experiment : We will conduct a simple experiment using spinach leaves to demonstrate that, in the presence of light and carbon dioxide, leaf tissues produce gas bubbles. While we cannot prove in this experiment that the bubbles are oxygen without a gas probe, we can demonstrate, by use of a control, that the bubbles only form when the leaves are submerged in a sodium bicarbonate solution (which releases CO 2 ) and not when they are submerged in pure water. We can also demonstrate that the bubbles only form in the presence of strong light, by moving the experiment into the dark and making further observations. Finally, we could experimentally vary the light intensity to demonstrate the effect of light intensity on the process.
When we dissolve baking soda (NaHCO 3 ) in water, carbonic acid (H 2 CO 3 ) and sodium hydroxide (NaOH) are formed. The carbonic acid then breaks down into water and carbon dioxide gas, which is why dissolving baking soda in water causes it to fizz.
NaHCO 3 + H 2 O --> H 2 CO 3 + NaOH
H 2 CO 3 --> H 2 O + CO 2 (gas)

- Fresh spinach leaves
- Metal paper hole punch
- 10 mL or larger plastic syringe (without needle) - get one from your local pharmacy
- Baking soda solution (dissolve some baking soda powder in water)
- Liquid dish soap solution (dissolve 5 mL in 250 mL of water)
- Cup 1: Detergent solution
- Cup 2: Baking soda solution (treatment)
- Cup 3: Water (control)
- Light source (fluorescent is good because it produces light without much heat)
- Use the metal hole punch to cut out 20 circular disks from the fresh spinach leaves, 10 for a control and 10 for a treatment.
- Separate the two parts of the syringe, drop 10 of the spinach disks inside, reassemble the syringe.
- Push the plunger almost to the bottom but don't crush the disks.
- Control or treatment
- For the treatment , draw up a small amount ~1 mL of detergent solution, and then draw the baking soda solution up to ~3-5 mL
- For the control , draw up a small amount ~1 mL of detergent solution, and then draw the water up to ~3-5 mL
- Point the syringe upward, tapping the sides, so that any air bubbles rise, and gently squeeze the syringe until liquid begins to come out.
- Put a finger on the end of the syringe, and draw the plunger back slightly, creating a partial vaccum.
- Repeat until the leaf disks are suspended in the solution. This action forces the liquid into the interior of the leaf.
- Watch this video of the process to make sure you're doing it right.
- Pour the contents of control and treatment syringes into two labelled clear plastic cups.
- Swirl the liquid to try to keep the disks from sticking to each other or the sides of the cups and then let them sit.
- Turn on a bright light, and monitor the disks every minute. Count how many disks are floating during each of the next 15 minutes.
- After all (or most) of the disks are floating, put the cups in the dark (a shoebox or a closet) and monitor for the next 15 minutes.
- Record how many disks remain floating after each minute until all (or most) of them have sunk.
Watch this demonstration to see how to make the leaf disks sink.
In the light, you should expect to see the disks in the control solution (water) stay on the bottom, but the disks in the treatment solution (baking soda) should begin to rise as they use the CO 2 to undergo photosynthesis and produce oxygen bubbles. The bubbles should cause the disks to float. After you remove the light and place the cups in the dark, the treatment disks should stop undergoing photosynthesis and the disks should begin to sink.
For comparison purposes, each lab group that does this procedure should report the time at which half (5) of the disks is floating. In the example below, that time would be about 11.5 minutes. You can use this Excel spreadsheet to record your data and it will auto-generate a graph for you.

Some or all of the submerged disks should begin to float within about 15 minutes
Questions :
- How does the suction help the leaf disks to sink?
- How does the detergent help the leaf disks to sink?
- Why don't the leaf disks soaking in the water (control) float?
- What is the purpose of the baking soda solution?
- What is the purpose of the light reaction?
- Why do the leaf disks in the baking soda solution (treatment) begin to float?
- Why do the leaves begin to sink again in the dark?
- Why don't the leaves in the baking soda solution continue to produce oxygen in the dark?
- Why do we use the half-way mark as a point of comparison rather than the point at which all the disks are floating?
- If the light-independent reaction can run without light, why does oxygen production (and presumably glucose production) stop?
References :
http://media.collegeboard.com/digitalServices/pdf/ap/bio-manual/Bio_Lab5-Photosynthesis.pdf
http://www.biologyjunction.com/5b-photoinleafdiskslesson.pdf
https://www.youtube.com/watch?v=XV9FOWleErA
http://www.berwicksclasses.org/AP%20Biology/Biology%20Assignments/AP%20BIOLOGY%20Lab%204.htm
http://www.kabt.org/2008/09/29/video-on-sinking-disks-for-the-floating-leaf-disk-lab/
Plants' Carbon Cycle: Death And Carbon Dioxide Release
- Last updated Nov 13, 2024
- Difficulty Intemediate
- Category Prune

Plants play a crucial role in the carbon cycle. They absorb carbon dioxide from the atmosphere during photosynthesis and store it in their roots, permafrost, grasslands, and forests. When plants die, they undergo a process called decomposition, where microorganisms break down the plant material and release the stored carbon back into the environment as carbon dioxide. This process of carbon cycling or carbon recycling ensures that the carbon within dead plants is not lost or locked away but instead is returned to the environment.
What You'll Learn
Plants release co2 during the day and night as part of the process of respiration, carbon is released into the environment through decomposition, the carbon cycle describes how carbon moves between the atmosphere, soil, living creatures, the ocean, and human sources, plants absorb co2 during photosynthesis and use it to produce sugars for food, as global temperatures increase, the amount of co2 released through plant respiration will increase.

Plants are essential for human survival, as they form the basis of natural ecosystems and absorb about 30% of all the carbon dioxide emitted by humans each year. They play a crucial role in the carbon cycle, which describes how carbon moves between the atmosphere, soils, living creatures, the ocean, and human sources.
During the day, when there is enough sunlight, plants perform photosynthesis, using carbon dioxide (CO2), water, and sunlight to produce sugars for food. However, plants also release CO2 during the day and at night as part of the process of respiration. Respiration releases energy from the plant's sugars, and as a result, plants release CO2 and water. Unlike photosynthesis, which occurs only in the green parts of the plant, such as leaves and stems, respiration occurs throughout the plant and happens continuously, day and night.
While plants release CO2 during respiration, the amount they release is not harmful to humans. In fact, plants are beneficial for human health and well-being, as contact with nature can help reduce negative feelings and boost positive ones. Additionally, caring for plants can be relaxing and rewarding.
As global temperatures rise, plants will release more CO2 through respiration. This increase in respiration could lead to a decline in the capacity of vegetation to absorb carbon emissions. However, it's important to note that plants also absorb more CO2 as levels rise, resulting in increased plant growth and productivity.
When plants die, the carbon within them is returned to the environment through decomposition. Microorganisms break down the plant material, releasing carbon back into the environment as CO2. This process is known as "carbon cycling" or "carbon recycling." Some of the carbon from dead plants may also eventually become fossil fuels over millions of years.
Respiration's Impact: Do Plants Lose Carbon Through Breathing?
You may want to see also
Decomposition is the process by which microorganisms break down plant material, converting organic carbon into carbon dioxide (CO2) through respiration. This CO2 is then released into the atmosphere or dissolved in soil water. This process is also known as "carbon cycling" or "carbon recycling".
The carbon cycle describes how carbon moves between the atmosphere, soils, living creatures, the ocean, and human sources. It is a closed system, meaning that the Earth does not gain or lose carbon overall. However, carbon is constantly moving between these reservoirs. Most carbon on Earth is stored in rocks and sediments, with smaller amounts found in the ocean, atmosphere, and living organisms.
Plants play a crucial role in the carbon cycle. They absorb carbon dioxide during photosynthesis and store much of this carbon in their roots, stems, and leaves. However, when plants die, this stored carbon is released back into the environment through decomposition.
Additionally, some carbon from dead plants may become buried underground before it has a chance to decompose. Over millions of years, this buried plant material can transform into fossil fuels such as coal, oil, and natural gas. When these fossil fuels are burned, the stored carbon is once again released into the atmosphere as CO2.
Plants' Scrubland Survival: Wetland Adaptations Explored
The carbon cycle is a process that moves carbon between plants, animals, and microbes; minerals in the earth; and the atmosphere. Carbon is the fourth most abundant element in the universe and is essential for life on Earth. It is a key component of DNA and proteins, and carbon in the form of carbon dioxide (CO2) helps regulate the Earth's temperature.
The carbon cycle can be divided into the fast carbon cycle and the slow carbon cycle. The fast carbon cycle, also known as the biological carbon cycle, operates in the biosphere and involves short-term biogeochemical processes. It can complete within years, moving carbon from the atmosphere to the biosphere and back again. The slow carbon cycle, also known as the geological carbon cycle, operates in rocks and can take millions of years to complete, moving carbon through the Earth's crust between rocks, soil, ocean, and atmosphere.
Plants play a crucial role in the carbon cycle by constantly exchanging carbon with the atmosphere. During the day, plants use sunlight, carbon dioxide, and water to produce sugars through photosynthesis. Plants absorb and store carbon dioxide in their roots, permafrost, grasslands, and forests. At night, plants release carbon dioxide through respiration as they convert sugars to energy. When plants decay or are burned, they release the stored carbon dioxide back into the atmosphere.
Animals and other organisms also contribute to the carbon cycle by exhaling carbon dioxide during respiration and releasing it during decomposition. The oceans absorb carbon dioxide from the atmosphere, which then sinks as the water cools. Over time, the absorbed carbon dioxide combines with calcium ions to form calcium carbonate, which settles on the ocean floor as limestone.
Human activities have a significant impact on the carbon cycle. Burning fossil fuels, wood, and other forms of carbon releases stored carbon into the atmosphere as greenhouse gases, disrupting the natural balance. Deforestation also affects the carbon cycle by removing carbon-storing plants and exposing soil that releases carbon through decay.
The carbon cycle is critical in shaping our climate. Carbon dioxide in the atmosphere acts as a greenhouse gas, trapping heat and regulating the Earth's temperature. Understanding the carbon cycle and our influence on it is crucial for the future of our planet.
How to Propagate Flowering Quince from Branch Cuttings
Plants are essential for human survival, as they form the basis of natural ecosystems and play a critical role in the carbon cycle. This cycle describes how carbon moves between the atmosphere, soils, living creatures, the ocean, and human sources. Carbon is a crucial element that enables the formation of complex molecules such as DNA and proteins, making life on Earth possible.
Plants are key players in the carbon cycle, constantly exchanging carbon with the atmosphere. During the day, when sunlight is available, plants engage in a process called photosynthesis. This is where they use carbon dioxide (CO2), water, and sunlight to produce sugars that they use as food. Plants absorb CO2 through openings called stomata, which also allow them to release moisture into the atmosphere.
Photosynthesis is a vital process for plants' energy and growth. The carbon dioxide absorbed during this process is used to produce carbohydrates and sugars, which serve as a food source for the plants. This process not only benefits the plants but also helps reduce the concentration of greenhouse gases in the atmosphere.
Plants capture carbon dioxide through photosynthesis and store it in their roots, stems, and leaves. However, when plants die, the carbon within them is returned to the environment through decomposition. Microorganisms break down the plant material, releasing carbon dioxide back into the atmosphere or soil water. This process of decomposition is often referred to as "carbon cycling" or "carbon recycling," highlighting the dynamic movement of carbon between different parts of the Earth system.
Blackberry Plants Blooming Season: Timing and Care Tips
Plants constantly exchange carbon with the atmosphere. During the day, when there is enough sunlight, plants use a process called photosynthesis to absorb carbon dioxide and water and, using sunlight, produce sugars to be used as food. Plants then use respiration to convert these sugars into energy. Respiration is a process that occurs constantly, both during the day and at night, and it releases carbon dioxide and water.
Plants are not immune to the effects of global warming. As global temperatures increase, so will the amount of carbon dioxide released through plant respiration. This is because respiration rates are directly linked to ambient temperature, with respiration rates peaking at an optimal temperature. As the temperature increases, so will the rate of respiration, as heat speeds up the reactions, increasing the kinetic energy.
The increase in respiration will have a significant effect on plant health and growth. As plants use more energy through respiration, they will need to absorb more carbon dioxide through photosynthesis to produce more sugars. This will impact the carbon cycle, as more carbon dioxide is released into the atmosphere and absorbed by plants. The carbon cycle is the process that moves carbon between plants, animals, and microbes; minerals in the earth; and the atmosphere.
The increase in global temperatures will also affect the amount of carbon dioxide absorbed by plants. Currently, plants take up and store around 25% of carbon emissions from the use of fossil fuels, helping to reduce the concentration of greenhouse gases in the atmosphere. However, as global temperatures rise, plants will begin to respire more, reducing their capacity to absorb carbon emissions. This means that the positive contribution of plants in reducing greenhouse gases may decline in the future.
Basil's Sunbathing Preferences: Grow Your Herb in the Right Light
Frequently asked questions.
Yes, plants release carbon dioxide when they die and decompose.
The process is called decomposition, where microorganisms break down the plant material and release carbon dioxide back into the environment.
The carbon cycle describes how carbon moves between the atmosphere, soils, living creatures, the ocean, and human sources. It is a closed system, meaning the Earth does not gain or lose carbon, but it is constantly moving.
Plants absorb carbon dioxide through a process called photosynthesis, where they use carbon dioxide, water, and sunlight to produce sugars for food and oxygen.
Yes, plants release carbon dioxide during the day and night through a process called respiration, where they convert sugars to energy and release carbon dioxide and water.

- James Turner Author

- Jeff Cooper Author Reviewer
It is awesome. Thank you for your feedback!
We are sorry. Plesae let us know what went wrong?
We will update our content. Thank you for your feedback!
Leave a comment
Prune photos.

Related posts

The Lucky Bamboo Companion: Care and Growth Guide
- Oct 22, 2024

Effective Deck Paint Removal: Saving Your Garden Plants
- Nov 11, 2024

Thai Chilli Plants: Sun Lovers or Shade Seekers?
- Nov 07, 2024

Kratom Plant Care: Why is My Plant Dying?
- Oct 25, 2024

The Best Bamboo Plants for Windowless Bathrooms
- Nov 09, 2024

Orchids: Outdoor Gardeners' Delicate Delights
- Nov 06, 2024
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 12 November 2024
Evidence for the acclimation of ecosystem photosynthesis to soil moisture
- Jinlong Peng ORCID: orcid.org/0000-0002-3038-6595 1 , 2 ,
- Jiwang Tang ORCID: orcid.org/0009-0009-9813-2186 1 , 2 ,
- Shudi Xie 1 , 2 ,
- Yiheng Wang 1 , 2 ,
- Jiaqiang Liao 1 , 2 ,
- Chen Chen 1 , 2 ,
- Chuanlian Sun ORCID: orcid.org/0000-0001-7130-0578 2 , 3 ,
- Jinhua Mao 1 ,
- Qingping Zhou 4 &
- Shuli Niu ORCID: orcid.org/0000-0002-2394-2864 1 , 2
Nature Communications volume 15 , Article number: 9795 ( 2024 ) Cite this article
1 Altmetric
Metrics details
- Climate-change ecology
- Ecosystem ecology
Ecosystem gross primary productivity (GPP) is the largest carbon flux between the atmosphere and biosphere and is strongly influenced by soil moisture. However, the response and acclimation of GPP to soil moisture remain poorly understood, leading to large uncertainties in characterizing the impact of soil moisture on GPP in Earth system models. Here we analyze the GPP-soil moisture response curves at 143 sites from the global FLUXNET. We find that GPP at 108 sites exhibits hump-shaped response curves with increasing soil moisture, and an apparent optimum soil moisture ( \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , at which GPP reaches the maximum) exists widely with large variability among sites and biomes around the globe. Variation in \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) is mostly explained by local water availability, with drier ecosystems having lower \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) than wetter ecosystems, reflecting the water acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) . This acclimation is further supported by a field experiment that only manipulates water and keeps other factors constant, which shows a downward shift in \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) after long-term water deficit, and thus a lower soil water requirement to maximize GPP. These results provide compelling evidence for the widespread \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) and its acclimation, shedding new light on understanding and predicting carbon-climate feedbacks.
Similar content being viewed by others
Atmospheric dryness reduces photosynthesis along a large range of soil water deficits
Soil moisture–atmosphere feedback dominates land carbon uptake variability
Increased photosynthesis during spring drought in energy-limited ecosystems
Introduction.
Terrestrial vegetation absorbs around one-third of anthropogenic carbon dioxide (CO 2 ) emissions through photosynthesis 1 , 2 . This is known as gross primary productivity (GPP), and represents the largest carbon flux between land and atmosphere 3 , 4 . It has been suggested that soil moisture critically influences plant photosynthesis 5 , 6 , yet accurately characterizing the impact of soil moisture on GPP is notoriously challenging in Earth system models 7 , 8 , leading to large uncertainties in assessing carbon-climate feedbacks 9 . A deep understanding of the response and acclimation of GPP to soil moisture is increasingly essential 5 , 10 .
In general, an increase in soil moisture can enhance GPP, as water is a primary limiting factor for plant photosynthetic processes 5 , 7 , 11 . As a result, most current models consider the stress of water deficit on GPP by implementing an empirical function ( β ) related to soil moisture 5 , 9 . The value of β ranges from 0 to 1, with β = 1 representing maximum photosynthesis without any soil moisture limitation 9 , 12 . Nevertheless, more and more recent studies observed a depressive effect of excessive soil water on GPP 13 , 14 , 15 , 16 , even in drylands that are typically water-deficient 8 . That depression is proposed to occur likely due to progressive soil anoxia and nutrient leaching, which restrict microbial and root metabolism and lower plant nutrient absorption, thereby impairing photosynthetic activity 17 , 18 , 19 , 20 , 21 , 22 . In this context, the GPP variations mediated by soil moisture should be expected as a unimodal (or hump-shaped) function with an apparent optimum soil moisture ( \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , corresponding to maximum GPP; Fig. 1 ). \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) indicates the transition of soil moisture effects on GPP from positive to negative. Despite this GPP-soil moisture framework is conceptually established 13 , 15 , the quantification of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) remains limited. To date, there has been no observation-based assessment of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) at the global scale. Even less is known about whether ecosystem GPP can acclimate to changes in soil moisture via shifting \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) . Recognizing these is pertinent to improve model performance in simulating carbon cycle and to develop climate change mitigation policies, given that most land areas are projected to experience a significant hydrological variability 5 , 23 , 24 .

The green vertical line indicates optimum soil moisture for GPP ( \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) ). An increase in soil moisture increases GPP when the soil moisture is below \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , but decreases GPP when the soil moisture is above \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) .
To quantify \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) directly, we use eddy covariance observations from FLUXNET datasets and derive the daily GPP-soil moisture response curves at globally distributed 143 sites. We then examine the relationship between soil moisture and \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) using eddy covariance observations, along with a field manipulation experiment. The complement of experiment is important, because it can systematically examine the causal effect of soil moisture on \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) . We address two central research questions: (i) whether \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) exists widely and (ii) how \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) changes with soil moisture.
Widespread presence of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\)
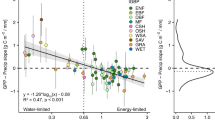
We determined \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) by fitting a concave quadratic model of soil moisture to GPP at daily scale, only when this model showed a superior performance (i.e., lower Akaike Information Criterion) than a saturation model (Supplementary Fig. 1 ; see Methods), along with statistically significant model parameters ( P ≤ 0.05; Supplementary Fig. 2 ). This detected presence of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) at 108 sites covering large areas and most vegetation types from a total of 143 sites in our dataset (Fig. 2 ; Supplementary Fig. 3 and Table 1 ). Across the 108 sites, \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) ranged from 5.8 to 56.9%, with median of 20.9%. We then selected a representative response curve from each vegetation type (Fig. 2b ), which indicated that GPP elevated with soil moisture but depressed when the soil moisture is above \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) . The paired t -test showed a non-significant difference between the Spearman’s correlation coefficients of soil moisture and GPP without and with controlling for air temperature, vapor pressure deficit, and incoming shortwave radiation (Supplementary Fig. 4 ; see Methods), suggesting that the air temperature, vapor pressure deficit, and incoming shortwave radiation had a negligible effect on the nonlinear relationship between GPP and soil moisture. Hence, the observed transition from an increase to a decrease in GPP with increasing soil moisture (i.e., the presence of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) ) was not caused by these confounding factors.

a Location of the 108 sites used in this study with detected \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) . The original map is from spData package in R . b Response of gross primary productivity (GPP, ln-scaled) to daily soil moisture at the 10 sites from 10 different vegetation types. The solid curve indicates the fitting of concave quadratic model, and the dotted line represents the detected \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) . Site name is shown in parentheses after each vegetation type. The vegetation types are as follows: CRO, croplands; CSH, closed shrublands; DBF, deciduous broadleaf forests; EBF, evergreen broadleaf forests; ENF, evergreen needle-leaf forests; GRA, grasslands; MF, mixed forests; OSH, open shrublands; SAV, savanna; WSA, woody savanna. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05.
Dependence of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) on soil moisture
We tested the relationship between growth soil moisture (SM growth , measured by average soil moisture during the peak growing season; see Methods) and \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) across sites using linear regression analysis. The results showed that \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) was positively correlated with SM growth across the 108 sites, with a linear regression slope of 0.92 (Fig. 3a ), and this relationship still held even when sites with a very strong GPP-soil moisture relationship were selected (Supplementary Fig. 5 ). It reflects the spatial acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change, and the slope indicates the acclimation pace. This acclimation was also observed across different vegetation types, with a slope of 0.75 (Fig. 3b ). Consistently, relative weight analysis further confirmed that \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) was majorly determined by SM growth (Supplementary Figs. 6 and 7 ), even accounting for other potential co-varying factors including air temperature, incoming shortwave radiation, vapor pressure deficit, soil temperature, total precipitation, leaf areas index, soil organic carbon, soil bulk density, soil sand fraction, soil cation exchange capacity, and soil pH.

a Linear regression of SM growth to \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) across 108 sites. b Linear regression of SM growth to \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) across vegetation types. Point with error bar indicates mean with standard error. Point size is proportional to the number of sites in each vegetation type. CRO, croplands, n = 10; CSH, closed shrublands, n = 2; DBF, deciduous broadleaf forests, n = 18; EBF, evergreen broadleaf forests, n = 9; ENF, evergreen needle-leaf forests, n = 29; GRA, grasslands, n = 21; MF, mixed forests, n = 7; OSH, open shrublands, n = 4; SAV, savanna, n = 5; WSA, woody savanna, n = 3. Solid line and shaded area indicate the linear regression fit and its 95% confidence interval respectively. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05.
We also revealed the relationship between \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) and SM growth at individual sites where at least 5 years of data were available to represent the temporal acclimation (Supplementary Table 2 ). We found that SM growth significantly increased \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) at temporal scale with median slope of 0.91, but its effect interacted with site, indicting geographically divergence in acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change (Fig. 4 a, b ). The relative weight analysis showed that precipitation was most responsible for this divergence (Supplementary Figs. 8 and 9 ; see Methods). Specifically, the stimulation of SM growth on \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) was larger in sites experiencing less precipitation (Fig. 4c ), implying that dry ecosystems could acclimate more quickly to water changes than wet ecosystems.

a Linear regression of SM growth to \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) across years at each site with ≥ 5 years of data (color lines). Each point represents one site-year data, with data from the same site being in the same color. SM growth , site, and SM growth ×site indicate the effects of SM growth , site, and their interaction on \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) respectively. b Probability density of the SM growth - \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) slope. The black dotted line is the median (0.91), and the gray dotted line is the zero. c Linear regression of precipitation (ln-scaled) to SM growth - \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) slope, with each point representing the slope at a site. Error bar represents the standard error on each slope. Solid line and shaded area indicate the linear regression fit and its 95% confidence interval respectively. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05.
To further verify the causal effect of SM growth on \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , we derived the GPP-soil moisture relationships and \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) at different precipitation levels in a field manipulation experiment (Fig. 5 a and b ; see Methods). We found that \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) showed significant increasing trend over increasing SM growth across precipitation treatments (Fig. 5c ), consistent with the eddy covariance results. At the same time, an increase in SM growth significantly decreased plant biomass allocation to belowground ( f BNPP ) (Supplementary Fig. 10 ). This plant parameter was significantly correlated with \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) (Supplementary Fig. 11 ), suggesting that SM growth shifted \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) mainly via changing f BNPP along the precipitation gradient in this experiment.

a View of the experimental plots. b Quadratic relationships between soil moisture and gross primary productivity (GPP, ln-scaled). The colored dotted lines indicate the detected \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) in differential precipitation levels (1/12P, 1/4P, 1/2P, 3/4P, P and 5/4P, where P was the annual precipitation). c Linear regression of SM growth to \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) across precipitation levels. Solid line and shaded area indicate the linear regression fit and its 95% confidence interval respectively. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05.
Several studies have shown examples of an apparent optimum soil moisture at which GPP reaches the maximum ( \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) ) 13 , 15 . However, it is still unclear whether \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) exists globally and what the underlying drivers are, impeding our understanding and predictive power of terrestrial biosphere feedback to a changing climate. We used global eddy covariance observations to detect the widespread \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) over large geographical regions spanning from 37.4° S to 71.6° N and 121.6° W to 148.2° E. We found that the distribution of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) was primarily shaped by local growth soil moisture (SM growth ) condition, with drier ecosystems having lower \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) than wetter ecosystems.
The widespread \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) indicates that the minimum GPP usually occurs at both soil moisture extremes, while the maximum GPP at moderate soil moisture. When exposed to water-deficient conditions, plants reduce their water loss to avoid hydraulic failure by closing stomata, thereby suppressing photosynthesis 25 , 26 . Drought can further restrict photosynthesis by enhancing photorespiration and then producing reactive oxygen species (ROS), which has been reported to damage the activity of essential photosynthetic enzymes (e.g., Rubisco) 27 , 28 . Excessive soil water limits plant photosynthesis possibly via aggravating soil anoxia that decreases the metabolic activity of plant roots and aerobic microorganisms (such as bacteria involved in ammonification and nitrification), and via inducing nutrient leaching that exacerbates nutrient limitation in plant photosynthetic processes 17 , 18 , 19 , 20 , 21 , 22 , 29 , 30 . Thus, the \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) arises from the intertwined effects of physical, physiological, and biochemical processes. Moreover, we acknowledge that the eddy covariance-observed transition from an increase to a decrease in GPP with increasing soil moisture might be caused not only by soil moisture itself, but also by other potentially confounding environmental factors (e.g., light and heat) 6 , 31 , 32 . But we found that the nonlinear relationship between soil moisture and GPP remained consistent after controlling for air temperature, vapor pressure deficit, and incoming shortwave radiation (Supplementary Fig. 4 ), suggesting that the existence of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) was unlikely caused by these confounding factors. This was further corroborated by a recent study showing that the correlation coefficient of soil moisture and GPP did not vary despite accounting for CO 2 and climate conditions 6 .
The tight link between \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) and SM growth potentially reflects the hydrological acclimation of vegetation. For example, when SM growth increases, plants upregulate their \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) in order to obtain more GPP returns. In contrast, when SM growth decreases, the downregulated \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) can alleviate plant damage caused by drought in terms of GPP loss. The explanation is that a decrease in SM growth starts to inhibit GPP only when the SM growth falls below \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , so a lower \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) means a higher threshold response to soil water deficit. To the best of our knowledge, this study is among the first to reveal the acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change.
We further found this acclimation to be scale-dependent. In particular, \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) generally showed a lower acclimation pace over time than across space. This suggests that spatial acclimation should not be conflated with temporal acclimation. The spatial acclimation reflects the ability of vegetation to cope with a changing environment through long-term changes in genetic material, community structure, and distribution over generations 33 , 34 , 35 . Nevertheless, the temporal acclimation is mainly based on reversible adjustments in plant morphology and physiology, as well as transient changes in ecosystem structure and functioning, without these long-term mechanisms 33 , 34 . Thus, the space-for-time substitutions proposed in previous studies could not be a valid approach for predicting vegetation acclimation to future climate change (e.g. 31 , 36 ), and continuous monitoring and long-term manipulation experiments are necessary 10 .
Interestingly, the temporal acclimation showed divergent paces among different sites, with precipitation being the dominant driver. The \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) exhibited a greater response to SM growth in sites with lower precipitation. This implies that soil drought would limit GPP more in wet than in dry ecosystems. Specifically, when SM growth decreases, a higher acclimation pace in drier ecosystems can result in a larger downregulation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , meaning a stronger resistance to drought. These findings challenge a popular hypothesis that arid and semi-arid ecosystems are more sensitive to soil water deficit than more mesic ecosystems 5 , 10 , 37 , 38 . Nevertheless, questions remain on what mechanisms accelerate the acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change in dry ecosystems. Further examination in future network control experimental studies is necessary, such as International Drought Experiment (IDE) 39 .
It should be noted that the acclimation observed by eddy covariance measurements was based on evidence of correlation between SM growth and \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , without indicating causal effect of SM growth on \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) . To this end, we further used a field experiment, which only manipulated water and kept other factors constant 40 . We found that there was a lower \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) in treatments with lower SM growth . Among the 8 biotic and abiotic factors examined, plant biomass allocation is shown to be the primary mechanism responsible for this acclimation. Specifically, decreased SM growth reduced \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) mainly by increasing belowground biomass allocation. The potential explanation is that a high biomass allocation to root system not only facilitates plant communities to directly access belowground water resources but also mitigates the water loss via reducing aboveground transpiration 22 , 41 , 42 , 43 , thereby downregulating soil moisture requirement to maximize photosynthesis. Taken together, these findings provide direct experimental evidence for the acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change. That being said, whether the mechanism proposed by our experiment is also responsible for the eddy covariance-observed acclimation remains unclear. We cannot further test this issue due to the lack of corresponding datasets in eddy covariance observations.
Our study sheds light on understanding and prediction of climate-carbon feedbacks at least in a couple of ways. First, the widespread \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) suggests that extremely low and high soil moisture both can inhibit plant photosynthesis. Unfortunately, most current Earth system models only consider the stress of water deficit on plant photosynthesis by implementing an empirical function related to soil water content 5 , 9 , without representing the depressive effect of excessive soil water, which could result in an overestimation of global GPP in models. Thus, we suggest that developing alternative models that account for the existence of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) has the potential to more realistically predict land carbon sequestration under climate change. Notably, this does not imply a complete rejection of the empirical function which does not consider \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , as there are still some sites where the concave quadratic model did not perform better than the saturation model (Supplementary Fig. 1 ). Second, the acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change has important implications for ecosystem resistance to soil drought. Previous studies that did not account for this acclimation likely exaggerated the negative effects of future drought on territorial carbon sinks (e.g. 5 ), especially for arid ecosystems that exhibit large acclimation pace. That being said, we only used the surface soil moisture data due to the limited measurements of deep soil moisture. Therefore, further investigation of the \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) and its acclimation is warranted in future studies that consider deeper soil moisture, especially in forest ecosystems, where plant roots are typically distributed at soil depths greater than one meter and have great potential to access groundwater resources 44 . Third, plant hydraulic or functional traits are critical in reflecting the acclimatation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change. As shown in our experiment, plants downregulated \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) under soil water deficit mainly by allocating more biomass to the root system.
In summary, our study provides compelling evidence for the widespread \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) and its acclimation to SM growth change using global eddy covariance observations and a field experiment. With decreasing SM growth , ecosystems tended to downregulate their \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , implying a higher threshold response to soil drought in terms of GPP loss. This acclimation would play a key role in stabilizing future land carbon sinks, as most areas are projected to have drier soils 5 . Our results also shed light on mechanisms by revealing the potential role of plant traits in driving the acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change. Given the importance of GPP in the global carbon cycle, our findings provide new insights for Earth system models to improve the predictive power of carbon cycling in the context of climate change.
Eddy covariance observations
To generate the GPP-soil moisture response curves, we used daily soil moisture and GPP from the eddy covariance observations provided in the FLUXNET datasets 45 , as the daily observations eliminate potential noise from the diurnal variation pattern of photosynthesis (such as midday depression in photosynthesis) 35 , 46 . The GPP estimates based on the nighttime partitioning method (i.e., GPP_NT_VUT_REF) were used 8 . Volumetric soil water content (%) was measured to determine soil moisture. The surface soil moisture data (SM_1: 0 to 10 cm, varying across sites) was used in this study, given that deeper soil moisture was only measured at a limited number of sites (<30) 47 . As plant responses to water variation may differ between growing and non-growing seasons, we focused only on the peak growing season, which is defined as June to August in the northern hemisphere and December to February in the southern hemisphere 6 , 48 . If >10% of 1-growing season data were missing from a site, that particular site-year was excluded from the analysis completely. We also excluded sites without soil moisture measurements and removed all wetland sites because these sites have a perched water table as well as infrequently exhibit soil moisture limitations 49 . These data filtering processes finally resulted in a total of 143 individual sites used in our study.
For each site-year, based on ordinary least squares, we determined \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) by fitting a concave quadratic model of soil moisture to GPP (Eq. 1 ), only when this model showed a superior performance (i.e., lower Akaike Information Criterion) than a saturation model (Supplementary Fig. 1 ), along with statistically significant model parameters ( P ≤ 0.05). The concave quadratic model has been commonly used in previous studies to detect the optimal photosynthetic environment at site or global scale (e.g. 13 , 31 , 50 ). The saturation model indicates that GPP increases as soil moisture increases in a manner consistent with the concave quadratic model when the soil moisture is below a certain threshold, but then levels off when the soil moisture is above this threshold (Supplementary Fig. 1 ), which is the similar representation of soil moisture stress effects on GPP in Earth system models 51 . Thus, compared to the saturation model, the observed superior performance of the concave quadratic model in fitting the data can be attributed solely to its consideration of the depressive effect of excessive soil moisture on GPP (i.e., the presence of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) ). We did not further consider other saturation models (e.g., exponential and sigmoid functions), as these models differ greatly from the concave quadratic model in their formula structure and it is thus unclear whether the observed superior performance of the concave quadratic model is really because it takes the depression of excessive soil moisture on GPP into account or simply because it fits the data better overall. The data quality (NEE_VUT_REF_QC, ranging from 0 to 1) of each observation was used as its weight in model 7 . Before fitting, GPP was logarithmically transformed (i.e., ln(GPP)), which is a commonly employed technique used in ordinary least squares to improve normality and minimize heteroscedasticity of the data 52 , 53 .
Where SM represents the soil moisture, \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) and GPP max are the vertex of the parabola, and a is parameter estimate of the fitted quadratic model and represents the direction and extent of curvature. Negative values of a mean that the curve is concave.
Considering that light and heat are potentially correlated or covariant with soil moisture 6 , we used partial Spearman’s correlation analysis and paired t -test to evaluate the impacts of air temperature, vapor pressure deficit, and incoming shortwave radiation when analyzing the GPP-soil moisture response curves. In particular, we first used the partial Spearman’s correlation analysis to estimate the strength of the nonlinear relationship between soil moisture and GPP while controlling for air temperature, vapor pressure deficit, and incoming shortwave radiation, as well as estimated it without controlling for any factors. We then used the paired t -test to test whether there is a significant difference between the Spearman’s correlation coefficients without and with controlling for air temperature, vapor pressure deficit, and incoming shortwave radiation. The results showed a non-significant difference (Supplementary Fig. 4 ), suggesting that the air temperature, vapor pressure deficit, and incoming shortwave radiation had a negligible effect on the nonlinear relationship between GPP and soil moisture. Thus, the observed transition from an increase to a decrease in GPP with increasing soil moisture (i.e., the presence of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) ) was not likely caused by these confounding factors. Among the 143 sites, GPP at 108 sites exhibited hump-shaped response curves with increasing soil moisture (Supplementary Fig. 3 and Table 1 ); we selected a site-year from each vegetation type to show a representative response curve (Fig. 2b ).
To identify the acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to soil moisture at spatial scale, we first averaged \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) across multiple years within each site. We then performed linear regression analysis to assess the relationship between \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) and average soil moisture during the peak growing season (SM growth ) across sites, and the acclimation pace was defined by the regression slope 31 , 35 . If an increase in SM growth does not result in a proportional increase in \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) (i.e., SM growth - \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) slope < 1), the increased SM growth would be more likely to suppress GPP once \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) falls below SM growth . Conversely, if an increase in SM growth results in a proportional or higher increase in \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) (i.e., SM growth - \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) slope ≥ 1), the increased SM growth would be more likely to stimulate GPP. We also conducted a relative weight analysis with the R package rwa to explore the potential effects of other climatic, soil and vegetation factors on the variability of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) across sites. The relative weight analysis considers collinearity among predictors, and partitions the explained variance across multiple predictors by transforming correlated predictors into orthogonal variables, performing a linear model on the transformed variables and then transforming the resulting coefficients back to the original metric 8 , 54 . The climatic factors included mean air temperature (Tem, °C), incoming shortwave radiation (ISR, W m −2 ), vapor pressure deficit (VPD, kPa), soil temperature (ST, °C), and total precipitation (Pre, mm) during the peak growing season from FLUXNET datasets. As vegetation structure variable, leaf areas index (LAI) for each site was extracted from global monthly LAI dataset according to its latitude and longitude 55 . All the above factors were averaged for each site over the years of observation. The soil properties included soil organic carbon (SOC, %), soil bulk density (BD, kg dm −3 ), soil sand fraction (Sand, %), soil cation exchange capacity (CEC, cmol kg −1 ) and soil pH (pH), which were directly retrieved from the Regridded Harmonized World Soil Database v.1.2 in the Oak Ridge National Laboratory Distributed Active Archive Center for Biogeochemical Dynamics.
To further get an understanding of the acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change at temporal scale, we revealed the relationship between \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) and SM growth at individual sites where at least 5 years of data were available (Supplementary Table 2 ). Before that, analysis of covariance (ANCOVA) was used to test whether this relationship was significantly different among different sites, with SM growth as the continuous variable and site as the categorical variable. Due to significant interaction between SM growth and site in the ANCOVA (Fig. 4 ), the relationship between SM growth and \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) across years was established in each site, indicting geographically divergence in temporal acclimation of \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to SM growth change (Fig. 4 ). To this end, we conducted a contribution analysis for this divergence among SM growth - \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) slopes using relative weight analysis, along with linear regression analysis (Supplementary Figs. 8 and 9 ). The potential contributors included Tem, ISR, VPD, ST, Pre, LAI, SOC, BD, Sand, CEC, and pH.
Field manipulation experiment
To verify the causal effect of SM growth on \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , we used a long-term precipitation manipulation experiment in an alpine meadow (32°50′ N, 102°34′ E, 3500 m a.s.l.). This experiment began in 2015, and consists of six precipitation levels: 1/12P, 1/4P, 1/2P, 3/4P, P and 5/4P, where P was the annual precipitation. There were five replications under each precipitation level, thus 30 plots (2 × 3 m) in total, following a fully factorial randomized block design. The experimental plots are presented in the Fig. 5a .
We conducted systematic measurements of ecosystem characteristics in 2022, during which precipitation treatments have significantly altered ecosystem structure and functioning as shown in He et al. 40 . For example, there were significant changes in soil moisture, species richness and dominance, and community stability along the precipitation gradient. These, in turn, would influence ecosystem water use capacity and potentially shift the response of GPP to soil moisture 40 , 56 , facilitating the capture of changes in the \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) under different precipitation treatments. Using a transparent static chamber (0.5 × 0.5 × 0.5 m) attached to an infrared gas analyzer (LI-6400, LI-COR, Lincoln, NE, USA), we measured GPP based on ecosystem CO 2 fluxes twice per month during the peak growing season (June–July–August) to reflect the temporal dynamics of GPP. Simultaneous with each measurement of GPP, soil moisture at a 0 ~ 10 cm depth in each plot was recorded for consistency with eddy covariance observations. Based on these measurements, we derived \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) in each precipitation level using the concave quadratic model (Eq. 1 ) as used for the eddy covariance observations. We then averaged soil moisture within each precipitation level to represent SM growht , and used linear regression to test the relationship between SM growht and \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) across precipitation levels. We also measured a serial of plant and soil properties that potentially regulate \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) to determine the mechanisms behind the effect of SM growht on \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) , including plant community height (CH), plant biomass allocation to belowground ( f BNPP ), and soil temperature (ST), total carbon (TC), total nitrogen (TN), pH, NH 4 + and NO 3 − . Pearson’s correlation analysis was applied to quantify the role of these properties in regulating \({{\rm{SM}}}^{{\rm{GPP}}}_{{\rm{opt}}}\) variation along SM growht . More detailed information on experimental site, design, and measurements can be found in the Supplementary Methods in the Supplementary Information.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
FLUXNET data are downloaded from https://fluxnet.fluxdata.org . Leaf area index data are obtained from https://daac.ornl.gov/cgi-bin/dsviewer.pl?ds_id=1653 . Soil properties are retrieved from the Regridded Harmonized World Soil Database v.1.2 in the Oak Ridge National Laboratory Distributed Active Archive Center for Biogeochemical Dynamics ( https://daac.ornl.gov/SOILS/guides/HWSD.html ). Experimental data generated in this study can be accessed at https://doi.org/10.6084/m9.figshare.27434715 .
Keenan, T. F. & Williams, C. A. The terrestrial carbon sink. Annu. Rev. Env. Resour. 43 , 219–243 (2018).
Article Google Scholar
Friedlingstein, P. et al. Global carbon budget 2021. Earth Syst. Sci. Data 14 , 1917–2005 (2022).
Article ADS Google Scholar
Beer, C. et al. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329 , 834–838 (2010).
Article ADS CAS PubMed Google Scholar
Anav, A. et al. Spatiotemporal patterns of terrestrial gross primary production: a review. Rev. Geophys. 53 , 785–818 (2015).
Green, J. K. et al. Large influence of soil moisture on long-term terrestrial carbon uptake. Nature 565 , 476–479 (2019).
Article ADS CAS PubMed PubMed Central Google Scholar
Wang, H. et al. Exploring complex water stress-gross primary production relationships: Impact of climatic drivers, main effects, and interactive effects. Glob. Change Biol. 28 , 4110–4123 (2022).
Article CAS Google Scholar
Zhang, Y. et al. Large and projected strengthening moisture limitation on end-of-season photosynthesis. Proc. Natl Acad. Sci. USA 117 , 9216–9222 (2020).
Kannenberg, S. A. et al. Dominant role of soil moisture in mediating carbon and water fluxes in dryland ecosystems. Nat. Geosci. 17 , 20 (2024).
Trugman, A. T., Medvigy, D., Mankin, J. S. & Anderegg, W. R. L. Soil moisture stress as a major driver of carbon cycle uncertainty. Geophys. Res. Lett. 45 , 6495–6503 (2018).
Knapp, A. K. et al. Field experiments have enhanced our understanding of drought impacts on terrestrial ecosystems-but where do we go from here? Funct. Ecol. 38 , 76–97 (2024).
Hu, C., Elias, E., Nawrocki, W. J. & Croce, R. Drought affects both photosystems in Arabidopsis thaliana . N. Phytol. 240 , 663–675 (2023).
Xie, S. D., Mo, X. G., Liu, S. X. & Hu, S. Plant hydraulics improves predictions of ET and GPP responses to drought. Water Resour. Res. 59 , 20 (2023).
Quan, Q. et al. Water scaling of ecosystem carbon cycle feedback to climate warming. Sci. Adv. 5 , 7 (2019).
Joshi, J. et al. Towards a unified theory of plant photosynthesis and hydraulics. Nat. Plants 8 , 1304–1316 (2022).
Article CAS PubMed PubMed Central Google Scholar
Zhang, J. W. et al. Water availability creates global thresholds in multidimensional soil biodiversity and functions. Nat. Ecol. Evol. 7 , 1002–1011 (2023).
Article PubMed Google Scholar
Zhang, T. et al. Analysis of the optimal photosynthetic environment for an alpine meadow ecosystem. Agr. For. Meteorol. 341 , 9 (2023).
Schuur, E. A. G. The effect of water on decomposition dynamics in mesic to wet Hawaiian montane forests. Ecosystems 4 , 259–273 (2001).
Schuur, E. A. G. Productivity and global climate revisited: the sensitivity of tropical forest growth to precipitation. Ecology 84 , 1165–1170 (2003).
Schuur, E. A. G. & Matson, P. A. Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 128 , 431–442 (2001).
Article ADS PubMed Google Scholar
Moyano, F. E., Manzoni, S. & Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 59 , 72–85 (2013).
Liptzin, D. & Silver, W. L. Spatial patterns in oxygen and redox sensitive biogeochemistry in tropical forest soils. Ecosphere 6 , 14 (2015).
Costa, F. R. C., Schietti, J., Stark, S. C. & Smith, M. N. The other side of tropical forest drought: do shallow water table regions of Amazonia act as large-scale hydrological refugia from drought? N. Phytol. 237 , 714–733 (2023).
Seneviratne, S. I. et al. Impact of soil moisture-climate feedbacks on CMIP5 projections: first results from the GLACE-CMIP5 experiment. Geophys. Res. Lett. 40 , 5212–5217 (2013).
Berg, A. et al. Land-atmosphere feedbacks amplify aridity increase over land under global warming. Nat. Clim. Change 6 , 869–874 (2016).
Medrano, H. et al. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 89 , 895–905 (2002).
Liang, X. Y. et al. Stomatal responses of terrestrial plants to global change. Nat. Commun. 14 , 13 (2023).
ADS Google Scholar
Miller, G., Suzuki, N., Ciftci-Yilmaz, S. & Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33 , 453–467 (2010).
Article CAS PubMed Google Scholar
Khorobrykh, S., Havurinne, V., Mattila, H. & Tyystjärvi, E. Oxygen and ROS in photosynthesis. Plant Basel 9 , 63 (2020).
Google Scholar
Franzluebbers, A. J. Microbial activity in response to water-filled pore space of variably eroded southern piedmont soils. Appl. Soil Ecol. 11 , 91–101 (1999).
Wu, Q. Q. et al. Contrasting effects of altered precipitation regimes on soil nitrogen cycling at the global scale. Glob. Change Biol. 28 , 6679–6695 (2022).
Huang, M. T. et al. Air temperature optima of vegetation productivity across global biomes. Nat. Ecol. Evol. 3 , 772–779 (2019).
Article PubMed PubMed Central Google Scholar
Chen, B. Z. et al. Inhibitive effects of recent exceeding air temperature optima of vegetation productivity and increasing water limitation on photosynthesis reversed global greening. Earth. Future. 10 , 12 (2022).
Smith, N. G. & Dukes, J. S. Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO 2 . Glob. Change Biol. 19 , 45–63 (2013).
Estiarte, M. et al. Few multiyear precipitation-reduction experiments find a shift in the productivity-precipitation relationship. Glob. Change Biol. 22 , 2570–2581 (2016).
Wang, B. X. et al. Dryness limits vegetation pace to cope with temperature change in warm regions. Glob. Change Biol. 29 , 4750–4757 (2023).
Niu, B. et al. Warming homogenizes apparent temperature sensitivity of ecosystem respiration. Sci. Adv. 7 , 11 (2021).
Huxman, T. E. et al. Convergence across biomes to a common rain-use efficiency. Nature 429 , 651–654 (2004).
Maurer, G. E. et al. Sensitivity of primary production to precipitation across the United States. Ecol. Lett. 23 , 527–536 (2020).
Smith, M. D. et al. Extreme drought impacts have been underestimated in grasslands and shrublands globally. Proc. Natl Acad. Sci. USA 121 , 10 (2024).
He, Y. L. et al. Long-term drought aggravates instability of alpine grassland productivity to extreme climatic event. Ecology 103 , 12 (2022).
Berendse, F. Competition between plant-populations with different rooting depths. 3. field experiments. Oecologia 53 , 50–55 (1982).
Zhou, X. H., Fei, S. F., Sherry, R. & Luo, Y. Q. Root biomass dynamics under experimental warming and doubled precipitation in a tallgrass prairie. Ecosystems 15 , 542–554 (2012).
Zeiter, M. et al. Timing of extreme drought modifies reproductive output in semi-natural grassland. J. Veg. Sci. 27 , 238–248 (2016).
Xiao, L. J. et al. Global depth distribution of belowground net primary productivity and its drivers. Glob. Ecol. Biogeogr. 32 , 1435–1451 (2023).
Pastorello, G. et al. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Sci. Data 7 , 27 (2020).
Zhang, Y. J. et al. Midday stomatal conductance is more related to stem rather than leaf water status in subtropical deciduous and evergreen broadleaf trees. Plant Cell Environ. 36 , 149–158 (2013).
Zhou, S., Zhang, Y., Williams, A. P. & Gentine, P. Projected increases in intensity, frequency, and terrestrial carbon costs of compound drought and aridity events. Sci. Adv. 5 , 8 (2019).
He, W. et al. Peak growing season patterns and climate extremes-driven responses of gross primary production estimated by satellite and process based models over North America. Agr. For. Meteorol. 298 , 16 (2021).
Fu, Z. et al. Critical soil moisture thresholds of plant water stress in terrestrial ecosystems. Sci. Adv. 8 , 12 (2022).
Dai, L. L. et al. Reduced photosynthetic thermal acclimation capacity under elevated ozone in poplar ( Populus tremula ) saplings. Glob. Change Biol. 27 , 2159–2173 (2021).
Article ADS CAS Google Scholar
Bonan, G. Climate Change and Terrestrial Ecosystem Modeling (Cambridge Univ. Press, 2019).
Band, N., Kadmon, R., Mandel, M. & DeMalach, N. Assessing the roles of nitrogen, biomass, and niche dimensionality as drivers of species loss in grassland communities. Proc. Natl Acad. Sci. USA 119 , 11 (2022).
Chen, L. Y. et al. Soil carbon persistence governed by plant input and mineral protection at regional and global scales. Ecol. Lett. 24 , 1018–1028 (2021).
Tonidandel, S. & LeBreton, J. M. RWA web: a free, comprehensive, web-based, and user-friendly tool for relative weight analyses. J. Bus. Psychol. 30 , 207–216 (2015).
Mao, J. & Yan, B. Global Monthly Mean Leaf Area Index Climatology 1981–2015. https://data.nasa.gov/dataset/ (2019).
Adams, M. A. et al. CO 2 , nitrogen deposition and a discontinuous climate response drive water use efficiency in global forests. Nat. Commun. 12 , 9 (2021).
Download references
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31988102, S.L.N.) and the National Key Technology R & D Program of China (2022YFF0802102, S.L.N.). We used the eddy covariance data of the FLUXNET community by the following networks: AmeriFlux, AfriFlux, AsiaFlux, CarboAfrica, CarboEuropeIP, CarboItaly, CarboMont, ChinaFlux, Fluxnet-Canada, GreenGrass, ICOS, KoFlux, LBA, NECC, OzFlux-TERN, TCOS-Siberia, Swiss FluxNet and USCCC. The ERA-Interim reanalysis data were provided by ECMWF and processed by Laboratoire des sciences du climat et de l’environnement (LSCE). The FLUXNET eddy covariance data processing and harmonization was carried out by the European Fluxes Database Cluster and the AmeriFlux Management Project (with support by European Union H2020 projects and the US Department of Energy Office of Science, respectively), with contributions from the Carbon Dioxide Information Analysis Center, ICOS Ecosystem Thematic Centre, and OzFlux, ChinaFlux and AsiaFlux offices. We also express our appreciation to Fangyue Zhang, Jiawei Shi, Chunxue Wei, Yifei Peng, and Zhangwei Gao for their help in field and laboratory measurements.
Author information
Authors and affiliations.
Key Laboratory of Ecosystem Network Observation and Modeling, Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing, 100101, China
Jinlong Peng, Jiwang Tang, Shudi Xie, Yiheng Wang, Jiaqiang Liao, Chen Chen, Jinhua Mao & Shuli Niu
College of Resources and Environment, University of Chinese Academy of Sciences, Beijing, 100049, China
Jinlong Peng, Jiwang Tang, Shudi Xie, Yiheng Wang, Jiaqiang Liao, Chen Chen, Chuanlian Sun & Shuli Niu
State Key Laboratory of Urban and Regional Ecology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085, China
Chuanlian Sun
Sichuan Zoige Alpine Wetland Ecosystem National Observation and Research Station, Southwest University for Nationalities, Chengdu, 610041, China
Qingping Zhou
You can also search for this author in PubMed Google Scholar
Contributions
S.L.N. supervised the study and wrote the manuscript draft. J.L.P. collected and analyzed the data, created graphs and tables, wrote the manuscript draft. J.W.T., S.D.X., Y.H.W., J.Q.L., C.C., C.L.S, J.H.M. and Q.P.Z. contributed to data analysis, interpretation, and wording. C.C. and J.Q.L. conducted the experiment and collected the data. All authors revised the manuscript.
Corresponding author
Correspondence to Shuli Niu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, peer review file, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Peng, J., Tang, J., Xie, S. et al. Evidence for the acclimation of ecosystem photosynthesis to soil moisture. Nat Commun 15 , 9795 (2024). https://doi.org/10.1038/s41467-024-54156-7
Download citation
Received : 09 April 2024
Accepted : 04 November 2024
Published : 12 November 2024
DOI : https://doi.org/10.1038/s41467-024-54156-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
During photosynthesis, plants convert light, water, and carbon dioxide from the air into oxygen and sugars. In this activity, you will be able to observe the oxygen production in leaves by doing a floating leaf disk experiment. You can also find out how quickly plants produce oxygen, and what variables affect photosynthesis.
Photosynthesis is a multi-step, enzyme-mediated process that converts light energy into chemical energy. During photosynthesis, plant cells use light energy (such as light emitted from the sun), water (H 2 O), and carbon dioxide (CO 2) as reactants to produce sugar molecules (C 6 H 12 O 6) and oxygen (O 2) (Figure 1):
At any given point in this experiment, the number of floating leaf disks is an indirect measurement of the net rate of photosynthesis. In photosynthesis, plants use energy from the sun, water, and carbon dioxide (CO 2 ) from the air to store carbon and energy in the form of glucose molecules.
The carbon dioxide in your breath will change the solution to a yellow color. Next, place leaves from kale into test tubes with the yellow solution. Place one tube in the light and another in the dark (using aluminum foil). A full spectrum grow light will have the best results. Leave one test tube empty as a control.
Photosynthesis is a multi-step, enzyme-mediated process that converts light energy into chemical energy. During photosynthesis, plant cells use light energy (such as light emitted from the Sun), water (H 2 O), and carbon dioxide (CO 2) as reactants to produce sugar molecules (C 6 H 12 O 6) and oxygen (O 2) (Figure 1):
Procedure. Prepare a bicarbonate solution by mixing 6.3 grams (about 1/8 teaspoon) baking soda in 300 milliliters of water. The bicarbonate solution acts as a source of dissolved carbon dioxide for photosynthesis. In a separate container, dilute a detergent solution by stirring a drop of dishwashing liquid in about 200 milliliters of water.
During photosynthesis, carbon dioxide (CO 2) from the atmosphere and water (H 2 O) from the soil is combined with light energy to produce glucose and oxygen (O 2). This process is essential for life on Earth, as it provides the basis for the food chain and produces the oxygen we breathe.
The process of photosynthesis is commonly written as: 6CO + 6H O → C 6 H 12 O + 6O. This means that the reactants, six carbon dioxide molecules and six water molecules, are converted by light energy captured by (implied by the arrow) into a sugar molecule and six oxygen molecules, the products. The sugar is used by the organism, and the ...
Some of the oxygen is used by the plant, but most of it is released into the atmosphere, to be breathed in by animals. In return, animals breathe out carbon dioxide, which plants use to create more oxygen (and food for the animals). Sunlight Oxygen is released Glucose is formed Carbon Dioxide Water Energy Chlorophyll Photosynthesis
Photosynthesis is powered by energy from sunlight. This energy is used to rearrange atoms in carbon dioxide and water to make oxygen and sugars. Carbon dioxide and water are inputs of photosynthesis. These inputs come from the environment. Oxygen and sugars are outputs of photosynthesis. The oxygen is released into the environment.
Plants extract the carbon dioxide from the air and use it in photosynthesis process to feed themselves. The carbon dioxide enters the leaves of the plant through small pores called stomata. Once the carbon dioxide enters the plant, the process begins with the help of sunlight and water. Advertisement. During this process, the plant combines ...
cycle representing both a large carbon pool, as carbon stored in plant biomass, and a large carbon flux in which plants take up car-bon (dioxide) with water and sunlight during photosynthesis. The Plant-A-Plant Class-room Experiments allow students to explore the necessary resources needed for plant growth and demonstrate how carbon dioxide (CO 2
Test both leaves for starch using iodine solution. Drop the leaf in boiling water. Transfer the leaf into hot ethanol in a boiling tube for 5-10 minutes. Rinse the leaf in cold water. Spread the leaf out on a white tile and cover it with iodine solution. Experiment to test if carbon dioxide is needed for photosynthesis.
In this experiment, you will. Use an O 2 Gas Sensor to measure the amount of oxygen gas consumed or produced by a plant during respiration and photosynthesis. Use a CO 2 Gas Sensor to measure the amount of carbon dioxide consumed or produced by a plant during respiration and photosynthesis. Determine the rate of respiration and photosynthesis ...
The stomata open to absorb the carbon dioxide needed to perform photosynthesis. They also open to release the oxygen produced by this process. Plant roots and leaves absorb water, which reacts with carbon dioxide using energy from light as the catalyst. Plant leaves are also able to absorb and release water through the stomata.
Photosynthesis separates carbon dioxide and water — known as CO2 and H2O, respectively — into their individual molecules and combines them into new products. Once the process is done, the plant releases Oxygen, or O2, into the surrounding air. It also creates C6H12O6, a substance similar to glucose, that feeds the plant.
Photosynthesis Lab. Photosynthesis is one of the most important anabolic chemical reactions that allows life to exist on Earth. With water, light energy from the sun, and carbon dioxide from the air, photosynthetic organisms are able to build simple sugars. Organisms that can make their own food are called autotrophs, and are at the base of the ...
During the day, when there is enough sunlight, plants perform photosynthesis, using carbon dioxide (CO2), water, and sunlight to produce sugars for food. However, plants also release CO2 during the day and at night as part of the process of respiration. ... Yes, plants release carbon dioxide during the day and night through a process called ...
carbon dioxide (CO2) from the air and combining it with water (H2O) and storing sunlight energy. The chemical formula is: 6CO2 + 6H2O + light Æ C6H12O6 (glucose) + 6O2 Notice that, in the process of building glucose, oxygen (O2) is given off. This is where the free oxygen on earth comes from. Prior to the evolution of photosynthesis, there was
Phytoplankton Emiliania huxleyi produce methane during photosynthesis, which may counteract their carbon sequestration potential, according to lab experiments on cultured phytoplankton.
Student's guide. otosynthesis and respirationPhotosynthesis is the energy-acquiring process in which light energy is used to produce oxygen, glucose and water f. om water and carbon dioxide. The reactions can. 6 O2 + C6H12O6 + 6 H2ONote that this is a simplified equation, and that the glucose produced by photosynthesis is immediately ...
Terrestrial vegetation absorbs around one-third of anthropogenic carbon dioxide (CO 2) emissions through photosynthesis 1,2.This is known as gross primary productivity (GPP), and represents the ...