A study of the rate of a candle burning
Michael P Jansen, Crescent School, Toronto, Ontario


Introduction
In this investigation, students will study the rate of burning of a candle as a function of the mass of the candle and as a function of the concentration, or partial pressure of O 2 (g).
Candle wax is a hydrocarbon (ca C 25 H 52 ). It combusts according to:
wax(s) + O 2 (g) → CO 2 (g) + HOH(g) (Equation 1)
(Wax burns as a vapour, but we indicate it as a solid; the mass of the solid is measured here.)
The rate law equation for this reaction is:
rate = k(solid wax) x [O 2 ] y (Equation 2)
In our lab we will burn the candle in air, in which [O 2 ] is constant. Therefore, equation 2 becomes
rate = k observed (solid wax) x , where k observed = k[O 2 ] y = constant (Equation 3)
Typical values for x are 0, 1 or 2, but higher integer values and even fractions are possible.
If x = 0, the reaction is zero-order with respect to the wax; If x = 1, the reaction is first-order with respect to the wax; If x = 2, the reaction is second-order with respect to the wax… you get the idea.
Later in this investigation students examine data for the combustion of a candle at increasing elevations above sea level, where the concentration (or partial pressure) of O 2 (g) is lower. This will allow students to determine the value of the exponent “y”.
To determine the rate of the reaction and the reaction order of wax(s) and of O 2 (g) using the equation rate = k[wax] x [O 2 ] y .
- small candle, such as a tea light
- small beaker
- electronic balance
- timing device
Safety precautions
Wear safety glasses; tie back hair and loose clothing. Make sure matches are extinguished before putting them in the garbage — not the sink.
Pre-lab questions or class discussion
- a) How do you expect that the rate of burning will vary with the mass (or length) of the candle? b) Based on your prediction in part (a) sketch the following graphs:mass of wax (y-axis) versus time (x-axis); burning rate (mass of wax per second) versus time.
Part 1. Determination of “x”
- Read the procedure and analysis questions before you begin. Use prepare a data table in which you record your findings and put calculated data. Place a heading at the top of each column.
- Place the tea light on an inverted small beaker. Ignite the candle, letting it burn for about two minutes in order to melt the wax near the wick.
- Start your timer and immediately record the mass of the candle. Record the mass of the candle every 60 s for at least five minutes.
Determination of “x” (Order of reaction with respect to solid wax)
- a) Plot a graph of mass of candle versus time. Use spreadsheet software to determine the equation of the line of best fit and the corresponding R 2 value.
- b) Plot a graph of [∆mass•time –1 in g•s –1 ] of the candle on the y-axis, versus time on the x-axis.
- a) Comment on the shape of your graphs. That is, are they linear/horizontal/vertical/parabolic/etc? b) Do your experimental results support your prediction? Explain briefly.
- What is the order of the reaction (the value of “x”) with respect to solid wax?
Sample results

Part 2. Determination of “y” (Order of reaction with respect to [O 2 ])
4. To determine how the partial pressure, which is related to concentration of O 2 (g) in the air, affects the rate of combustion of a candle, use the data below to plot a graph of combustion rate (g•min –1 ) versus PPO 2 — use spreadsheet software. The data were obtained by Crescent School students on the slopes of Mount Kilimanjaro (outreach trips to Tanzania). They burned candles at increasing elevations above sea level. (Since air is 21% O 2 by volume and by pressure, the partial pressure of O 2 can be obtained by multiplying the atmospheric pressure (P atm ) by 0.21.)

The following table has the combustion rate of a candle at increasing elevation above sea level. Partial pressure of O 2 can be calculated and filled in.
5. What measurements did the students who obtained these data record?
6. Plot a graph of rate of wax combustion (y-axis) versus PPO 2 . From your graph determine as quantitatively as possible, the effect of the PPO 2 on reaction rate.
7. What is the order of the reaction with respect to PPO 2 ?
8. Rewrite Equation 2 with suitable values for the exponents.

Toronto, Canada is at 76 m, typically around 102 kPa. In this analysis we assumed that the temperature of the burning candle is the same at the different altitudes. If this is not the case, our rate data collected on the mountain slope would reflect both the effect of a (presumably) decreasing flame temperature and the decreasing partial pressure of oxygen.
More about February 2019

Department of Chemistry 200 University Ave. W Waterloo, Ontario, Canada N2L 3G1
All Science Fair Projects
1000 science fair projects with complete instructions.

Burn Rate of a Candle
Science fair project description.
A candle in a colder room will burn more slowly.
Candles Candles are used to provide light when there is no electricity available or during power failures. Candles are also used to create a romantic setting at restaurants. Most candles are made of paraffin wax. Candles are also made from other types of materials such as soy wax, bee wax, plant wax or animal fat. The wax in a candle is used as fuel to keep the candle burning. The wick used in a candle is an absorbent twine and must have strong capillary action. When the wick is lit, the wax around the wick will melt due to the heat of the flame. The capillary action of the wick will absorb the melted wax and pull it upwards. When the wax is exposed to the flame, it will vaporize and the wax vapor burns. The wick does not burn as quickly, because the vaporizing wax will help to cool the temperature of the wick and protect it. Candles are manufactured in various sizes, scents and colors. Some of the scents used in candles include flower scents, fruit scents, vanilla and even green tea. These scented candles are used in aromatherapy and spas. Candles are also used in religious ceremonies and of course, what's a birthday cake without candles?
Scientific Terms
The materials required for this science fair project: - 15 candles. The candles should be of the same diameter, length and be made by the same manufacturer - A ruler - A caliper - A stopwatch - A match box or lighter - A room with air-conditioning (preferably reverse-cycle) - A heater (if reverse-cyle air-conditioning is not avaiable) - A thermometer
1. For this science fair project, the independent variable is the temperature of the room, i.e. 20°C, 25°C and 30°C. The dependent variable is the length of the candle after it has burned for 30 minutes. This is determined by measuring the length of each candle with a ruler before it is lit and after it has burned for 30 minutes. The constants (control variables) are the type and length of the candle, the diameter of the candle and the time the candle is allowed to burn. 2. Using the ruler and caliper, the length and the diameter of the 15 candles are measured for consistency. If the measurement of a candle deviates by more than 1 millimeter from the average, have that candle replaced. All 15 chosen candles are labeled as “A1” to “A5”, “B1” to “B”5 and “C1” to “C5” respectively. The length of the 15 candles is measured and recorded. 3. The air conditioner in the room is switched on and the room is left to is cool, until the room temperature measures 20°C. The temperature of the room is verified with the thermometer. The candles marked “A1” to “A5” are lit in the room and the stopwatch is started. Ensure that windows are closed and the candles are not placed anywhere near the air-conditioner blower (avoid drafts). After 30 minutes, the flames on the candles are extinguished and the length of each candle is measured again with a ruler. The reduction in candle length is calculated and recorded in the table shown below. You may need to air the room to get rid of the candle smoke, before proceeding to step 4. 4. The air conditioner is switched off and the room temperature is allowed to rise to 25°C. You may need to turn on the heater to achieve this temperature. The temperature of the room is verified with the thermometer . Procedure 3 is repeated using the candles marked “B1” to “B5”. The reduction in candle length after 30 minutes is calculated and recorded in the table shown below. You may need to air the room to get rid of the candle smoke, before proceeding to step 5. 5. The heater is turned on in the room until the room temperature reaches 30°C. The temperature of the room is verified with the thermometer. Procedure 3 is repeated with candles marked “C1” to “C5” and the reduction in the candle length after 30 minutes is calculated and the results recorded. in the table below.
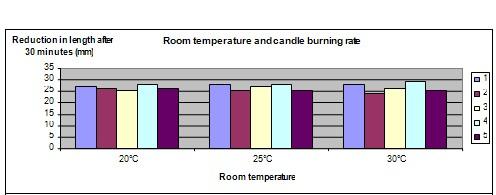
The length of the burnt away in 30 minutes is almost the same at all 3 different room temperatures.
The graph below represents the results of our science experiment:
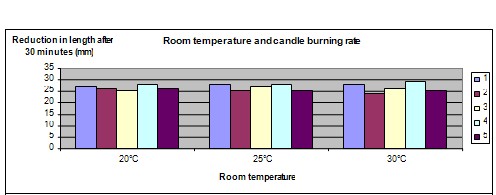
The hypothesis that a candle in a colder room will burn more gradually/slowly is disproved. All of the candles burned at almost the same rate regardless of the surrounding temperature.
Also consider
This science fair project can also be varied to compare the burn rate of candles made from different materials like soy wax or animal fat. Try to repeat the experiment to compare the weight of the candle instead of the length. Would there be a difference? What if the room temperature were to be varied more significantly? Eg. Measuring burn-rate at 5 degrees, 20 degrees and 40 degrees?
Candle - http://en.wikipedia.org/wiki/Candle How does a candle work? - http://home.howstuffworks.com/question267.htm
Related videos
Related Science Fair Project Ideas
Science Project Ideas

Do White Candles Burn Faster Than Colored Candles
Introduction.
This science fair project topic that tests white candles vs colored candles with respect to how long they take to burn requires commonly available supplies and can be performed easily. You can apply the knowledge gained from your day-to-day activities involving burning candles. Background information on the project would come in handy during the execution. Plotting a graph of the different rates at which the candles burn helps in the discussion.

Do Colored Candles Burn Faster Than White Candles Project
Problem statement.
The experiment proves which candle burns faster, white or any of the colored ones.
The experiment would establish the fact that white colored candles burn quicker than white candles due to the chemicals present in them.
Candles of different colors and white are burned up to a specific mark on their bodies and the time taken is checked to analyze which one burns the fastest.
- White and colored candles of the same brand, same size and shape
- Permanent marker
- Trim the wicks of the candles such that all are of the same length and place them about 10 inches apart on a table so that the burning of any candle doesn’t affect the adjacent one.
- Mark a line on the candle that is one inch from the top with the permanent marker.
- Light them simultaneously and observe the time it takes to burn up to the mark.
- Record the observation data in a table format as given below.
- Plot a graph (a bar graph would be good) with the color as the X-axis (independent variable) and the time taken to burn up to the mark as the Y-axis (dependent variable). You can prepare a proper lab report and charts too.
All the colored candles take times that are lesser than the white candle. Note that the size and brand of the candles are constant and hence they are the controlled variables
Result/Conclusion
The white candle burns slower than the colored candles.
Explanation
The colored candles are seen to burn less than the white ones as the colored ones have dyes that are nothing but chemicals which make the candles hotter and burn them quicker.
Background Research
The candles are nothing but a mass of wax with a wick in the middle. Chemical compounds are mixed together to make wax the major ones being beeswax and paraffin wax. The wick is a piece of braided cotton the thickness of which, apart from the wax, also decides the time taken for a candle to burn.
There can be different kinds of candles such as tea light, taper and jar candles. Floating candles can be put in water while outdoor candles are the bigger varieties. There are the scented types and the birthday candles are skinny. With the advent of electricity, candles are now mostly used for emergency purposes.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
REPORT ON CANDLE BURNING EXPERIMENT

This is a short report that summarizes an experiment about the heat produced from the combustion process of a candle. The main objective of this report is ∗ to give details of how the data of the experiment is collected, ∗ data transformation, ∗ model fitting, ∗ comparison of the two fits, ∗ and logical interpretations for each step.
Related papers
Journal of Fire Protection Engineering, 2005
Common household open flame and radiant ignition sources are the actual or suspected cause for many fires. The purpose of this research is to identify the burning behavior and properties of common candles in order to provide additional tools for use by fire investigators. The properties of paraffin wax are obtained from the literature and from experiments. The candles are burned under controlled laboratory conditions to measure the mass burning rate, candle regression rate, flame height, and heat flux. Using the properties of paraffin wax and characteristics of the candles, numerous simulations are performed with the NIST Fire Dynamics Simulator (FDS) to model the burning rate and heat flux profile of the candle flame. The modeling results are then compared with the flame height and heat flux data obtained experimentally. The model facilitates an enhanced understanding of the structure of candle flames.
In this book, we develop the governing equations of heat flow that explain the experimental observations. We look at the heat combustion up a wick in paraffin and we use the Darcy law for flow in porous mediums to explain the observed. We go ahead to explain what is observed for combustion of a candle. After that we go ahead and explain how gravity affects the rate of combustion of a candle and paraffin up a wick. We quantify also all the other factors that affect the rate of combustion. Other phenomena are also explained.
Heat of Combustion, 2022
The purpose of this study is to calculate the heat of combustion using two distinct types of alcohol: isopropyl alcohol and ethanol. The experiment was carried out with a variety of alternative materials. Tables are provided to illustrate the calculated numerical value of the experiment performed using the specified fuels. The analysis revealed that, in comparison within each stated fuel, isopropyl has the least quantity of consumption as the fuel when the freshwater temperature reaches 20°Celsius. As it is utilized in the combustion reaction, the mass of the fuel decreases. Moreover, the temperature of the water rises due to the combustion of the fuel, which generates energy that heats the water.
Science & Education, 2011
The experiment in which a candle is burned inside an inverted vessel partially immersed in water has a history of more than 2,200 years, but even nowadays it is common that students and teachers relate the change in volume of the enclosed air to its oxygen content. Contrary to what many people think, Lavoisier concluded that any change in volume in this experiment is negligible; moreover, the explanation relating oxygen consumption in the air with its change in volume is known to be wrong. In this work we briefly review the history behind the candle experiment and its relationship with some typical erroneous explanations. One of the key factors behind Lavoisier's success was the use of experiments carefully designed to test different hypotheses. Following these steps, we performed several closed volume experiments where the candle wick was replaced by a capillary stainless steel cylinder supported and heated by a nichrome filament connected to an external power supply. Our recorded experiments are displayed as web pages, designed with the purpose that the reader can easily visualize and analyze modern versions of Lavoisier's experiments. These experiments clearly show an initial phase of complete combustion, followed by a phase of incomplete combustion with elemental carbon or soot rising to the top of the vessel, and a final phase where the hot artificial wick only evaporates a white steam of wax that cannot ignite because no oxygen is left in the closed atmosphere. After either a complete or incomplete combustion of the oxygen, our experiments show that the final gas volume is nearly equal to the initial air volume.
RECENT RESEARCH ACTIVITIES ON CANDLE BURNUP. A new reactor burnup strategy CANDLE (Constant Axial shape of Neutron flux, nuclide densities and power shape During Life of Energy producing reactor) was proposed in 2000, where shapes of neutron flux, nuclide densities and power density distributions remain constant but move upward (or downward) along its core axis. Since then many research activities have been performed in Tokyo Tech. In this paper these activities are summarized, and some other important activities relating CANDLE burnup performed in the other institutes in the world are also introduced.
A small candle flame was characterized using a standard light extinction technique and the classical CH* chemiluminescence measurements. Mass loss rates were estimated using an image treatment method. Soot volume fractions, radiative emission and mass loss rates were measured. The results agree well with other published laminar diffusion flames. View factor calculation was carried out in order to estimate the radiate fraction of the flame using two methods, a numerical one using a more complex geometry to model the flame and an integrated result considering a simple geometry. Results agree well, which might justify the use of simplified geometries in fire safety engineering practice, particularly for the far field radiative transfer calculations.
Research Papers Faculty of Materials Science and Technology Slovak University of Technology, 2017
The paper deals with the assessment of selected fire safety characteristics of candles. Weight loss of a candle during the burning process, candle burning rate, soot index, heat release rate and yield of carbon oxides were determined. Soot index was determined according to EN 15426: 2007 - Candles - Specification for Sooting Behavior. All samples met the prescribed amount of produced soot. Weight loss, heat release rate and the yield of carbon oxides were determined for one selected sample. While yield of CO increased during the measurement, the yield of CO
for their valuable suggestions. contributions and help to this study and to Ken Hom in assisting the experiments. The author wis to thank Mrs. Carmen Hubbard for her expert typing and Mrs. Gloria Pelatowski for her effort in rendering the graphs. The author would also like to thank the residents of Hesse Hall for their moral support. and finally, his parents for their understanding and encouragement in support of the achievement of this goal.
Experimental Thermal and Fluid Science, 2017
Soot volume fractions and soot temperatures have been measured for the first time on candle flames. Measurements on laminar steady flames were carried out using candles with wick diameters of 2, 3 and 4 mm. Wick length was varied between 4 and 10 mm. The shape of the candle flame was obtained from CH ⁄ spontaneous emissions. Measured flame heights show an increase with wick dimensions, approaching an asymptotic value for increasing wick lengths. Soot volume fractions were obtained from laser extinction measurements with the Modulated Absorption/Emission (MAE) technique. A deconvolution technique and a regularization procedure were applied to the data. Radial profiles of soot volume fractions increase when varying the wick dimensions; this effect is produced by the greater amount of fuel released by the wick. Radially integrated soot volume fractions were also calculated, presenting a similar behavior to the soot volume fraction radial profiles. The peak integrated soot volume fraction was found at approximately half the flame height, independent of the wick dimensions and burning rates. Soot temperature was obtained from emission measurements at two different wavelengths considering the attenuation of the soot particles in the optical path length. A deconvolution and regularization procedure was carried out in order to obtain temperature profiles for different heights in the flame. The observed increase in soot production and soot temperature profiles was directly related to the higher burning rate experienced by the candle. The results show that peak integrated soot volume fractions are proportional to both the mass loss rates and the flame heights.
PSYCH up2date, 2020
ZENITH International Journal of Business Economics & Management Research, 2013
AMBIENTE E DIRITTO: DOGMI MODERNI, PRASSI ANTICA, 2023
BMC Palliative Care, 2022
Journal of Research in Applied Linguistics, 2023
Middle East Forum, 2024
Leiden Journal of International Law, 2010
International Journal of Pediatric Endocrinology, 2011
Advances in Fuzzy Systems, 2009
PLOS Computational Biology, 2017
Nigerian journal of medicine : journal of the National Association of Resident Doctors of Nigeria, 2010
MMWR. Morbidity and mortality weekly report, 2015
Neurocomputing, 2000
ADSU Journal of Accounting Research (AJAR), Vol.3 (1& 2) (December) Pp 70-79, 2014
The Journal of Experimental Biology, 1991
Related topics
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Burning Questions About a Candle
Introduction.
Yogi Berra once said, "You can observe a lot by just watching." What he meant by this is that observation requires more than simply using your eyes . It also requires critical thinking during the observation process. You should be asking yourself questions about what you are seeing and why the behavior you are observing is taking place. That is, you should be formulating hypotheses and testing those hypotheses on a limited basis. This type of observation is extremely important in science since it provide the main method of acquiring data, detecting errors and unexpected occurrences, and achieving ultimate success in whatever component of science you are engaged in.
The key aspect of critical observation involves self-questioning. That is, you must learn to formulate questions based on what you are seeing that you can either answer immediately or attempt to answer at a later time. This type of question/answer dialogue, carried out internally, provides the mental aspect to the observation process which is so crucial in extending the data acquisition to understanding and application. (For example, see this note on the discovery of penicillin.)
Are you ready to test your powers of observation by answering...
Activity: Observations of a Burning Candle
Materials needed.
Attached here is a printable form that has most of the questions and directions below, but with space for you to write in your answers and observations while you work. Have fun!
Observations to make before you light the candle
Answer the following questions concisely . That is, use short sentences or sentence fragments, and only say what really needs to be recorded. You may want to work in pairs or small groups, each writing down observations, then periodically comparing notes and asking each other questions. If you do it this way, just remember: don't make critical comments! Be critical in your thinking, in the sense of always asking yourself "What does that mean anyway?" and not in the sense of "That's clearly wrong" or "What a dumb observation." Be positive in your approach, focus on the candle, and keep asking yourself questions in your own mind, especially questions that lead to further observations and more questions.
Do some physical "tests" on the candle using what you have on you or laying around the room. What do these tests tell you about its properties?
Do any of these tests give you an indication of the nature of the candle components at the molecular level? What do you know about the chemical nature and/or composition of these components?
Hypothesize what will happen when you light the candle and start it burning
Now, light the candle and watch what happens.
- It is important that you record your observations as quickly as you can and then come back to some of them later for further thought and extended observation.
- Try to make at least 10 distinct and separate observations initially. (Be aware that individual observations numbering over 100 have been recorded by others.)
It may take you a while to begin to think about what is happening. Be patient and work at the process. Once you begin to see how to ask questions related to what you are seeing, a snowball effect will occur that should open up whole new areas of observation for your critical consideration. Be sure to write down all of your thoughts and observations as they occur. Don't try to be critical of the material you write down, but simply put it down as quickly as possible. You will have plenty of opportunity later for evaluation and grouping of your data.
Take one observation that you recorded above and:
- Break it down into macroscopic versus molecular levels.
- See if you can coordinate what is happening between what you've physically observed (that is, the macroscopic level) and the molecular level.
- Most important, speculate on what is actually happening at each of these levels.
- What changes in that property occurred during the burning process? Go into as much detail as you can.
Now, pick another observation or two and carry out the same level of in-depth analysis. Try to relate the observations you made above to those that you make in this section.
You should find that as you practice this process of observation and recording your thoughts, you become better at asking questions about what is happening, and then writing down answers. This iteration is part of "doing science."
Repeat this process several times: this repetition is also part of doing science.
Now try to "watch" yourself observing and asking yourself questions:.
- How can you do this better, with conscious awareness?
- Can you see possible ways of getting the answers while you are asking the questions?
Test Your Hypothesis by Doing an Experiment
After you feel like you have made enough observations to give you a pretty good understanding of what is actually happening during the burning process, it is time to do an experiment . An experiment results when you ask a question and then develop a method to answer that question. The experiment itself is actually the process of getting the answer.
- First, try to ask a good question. Think about the observations that you made, especially those that you don't really understand, and try to formulate some kind of hypothesis (or good guess) about what is happening.
- Once you have a good question, try to think of ways that you can answer that question. What kind of measurements or observations (either with your own eyes or with other methods) would help you get the answer? Think about the kind of data you'll collect - will your data be qualitative (that is, descriptive) or quantitative (with numerical measurements). What do you have available in the lab that you could use to measure changes that occur?
- Once you have some idea of how to actually do the experiment, go ahead and do it.
- Safety First! Make sure that you consider safety at every step. (Note: NEVER put a thermometer into the flame. The high temperature of the flame will break most thermometers.)
- As you design your experiment, consider how you will present your results. A good experiment is reproducible, and so you'll need to outline your steps (procedure) very specifically. Don't get bogged down in making it perfect the first time around, just make sure that you take clear and specific notes.
Be creative - you should be able to design experiments that you can do with what you can find in the lab or that you can easily acquire with very little effort. For example, holding a piece of glass over the candle as it burns (probably at an angle would work better) can help you answer questions about what is evolved or given off during the combustion process. Think of other simple ways of investigating further what is going on and see what kind of good experiments you can come up with.
A Burning Candle: An Experiment in Observation From the Macrogalleria
Candle Flames From NASA's Microgravity: A Teacher's Guide with Activities, Secondary Level
Burning Questions about a Candle written by Lon Mathias, from http://pslc.ws/pslcweb/activity/candle/candle3.htm
Michael Faraday (1791-1867): The Chemical History of A Candle, 1860 From the Internet Modern History Sourcebook
- "The Persistence of the Candle-and-Cylinder Misconception," by James P. Birk and Anton E. Lawson, Journal of Chemical Education, 1999, 76, 914-916. This article discusses why a candle under an inverted beaker in a container of water does not indicate the percent of oxygen in the air. The following is an excerpt from this article: "In conclusion, in every experiment that purports to measure 21% oxygen in air by a combustion process, combustion is not consuming all the oxygen. Rather, the heating of air results in partial expulsion of air. The flame is extinguished by local depletion of oxygen and buildup of carbon dioxide, not by complete combustion of oxygen in the remaining air. When the flame expires, the apparatus and enclosed air cool back to room temperature, causing a pressure differential that results in a rise in the water level."
"'Experiment with a Candle' without a Candle," by Dusan Krnel and Sasa A. Glazar, Journal of Chemical Education, 2001, 78, 914.
Other articles that might be of interest:
- "A new use for the candle and tumbler myth," by Gavin D. Peckham, J. Chem. Educ. , 1993 70 1008.
- "Demonstration of the burning of a candle," by Isidor S. Hirschhorn, J. Chem. Educ. , 1941, 18, 107.
- "Evolution of the candle," by G. Griffin Lewis, J. Chem. Educ. , 1934, 11, 367.

IMAGES
COMMENTS
Candle burning investigation. By Tim Jolliff. No comments. ... They will need experimental data in order to test their prediction. ... This could be used to follow on from a class investigation into the effect of beaker size on the length of time the candle burnt. The slides can be used to guide a class discussion, in combination with or as an ...
Use prepare a data table in which you record your findings and put calculated data. Place a heading at the top of each column. Place the tea light on an inverted small beaker. Ignite the candle, letting it burn for about two minutes in order to melt the wax near the wick. Start your timer and immediately record the mass of the candle.
The experiment was done by measuring how much of a candle will melt in 30 minutes when placed in an environment with surrounding temperatures of 20°C, 25°C and 30°C. ... The wax in a candle is used as fuel to keep the candle burning. The wick used in a candle is an absorbent twine and must have strong capillary action. When the wick is lit ...
The experiment proves which candle burns faster, white or any of the colored ones. Hypothesis. The experiment would establish the fact that white colored candles burn quicker than white candles due to the chemicals present in them. Abstract. Candles of different colors and white are burned up to a specific mark on their bodies and the time ...
This is a short report that summarizes an experiment about the heat produced from the combustion process of a candle. The main objective of this report is ∗ to give details of how the data of the experiment is collected, ∗ data transformation, ∗ model fitting, ∗ comparison of the two fits, ∗ and logical interpretations for each step.
The candle apparatus protects the candle from drafts. 2) Mass the candle and large can to the nearest 0.01 gram. Record the mass of candle and can in your data table. Note the balance so that when you mass the candle again you can use the same balance. 3) Fill a beaker with approximately 300 mL of water from the cold water tank / cooler. Do not ...
The "burning candle" experiment ( (Massalha, 2016; Massalha, Thimor and Gluck;, is used at the junior high school program to prove that there is a component in the air, which is essential to the ...
For each experiment, four candles were burned in the chamber simultaneously, thus a mean value was therefore measured for each individual candle type by averaging the differing emission and burning behaviors. ... The concentration versus time behavior for various online measured parameters is shown in Fig. 3 using the data from the candle type ...
Burning Questions About a Candle Activity: Observations of a Burning Candle. Purpose This experiment will introduce you to observations involving critical thinking plus use of your notebook to record observations before report writing. Your teacher will describe the type of laboratory report that will be required. Materials Needed. Candle
Wax acts as a fuel to keep the candle burning. The wick has to be an absorbent twine with strong capillary ability. All candles used in this experiment are of the same height, size, shape and brand. Research Question: Is the burn rate …show more content… The processed data validates the data analysis and supports to form a stronger conclusion.