An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


5G Wireless Communication and Health Effects—A Pragmatic Review Based on Available Studies Regarding 6 to 100 GHz
Myrtill simkó, mats-olof mattsson.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Received 2019 Aug 19; Accepted 2019 Sep 11; Issue date 2019 Sep.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/ ).
The introduction of the fifth generation (5G) of wireless communication will increase the number of high-frequency-powered base stations and other devices. The question is if such higher frequencies (in this review, 6–100 GHz, millimeter waves, MMW) can have a health impact. This review analyzed 94 relevant publications performing in vivo or in vitro investigations. Each study was characterized for: study type (in vivo, in vitro), biological material (species, cell type, etc.), biological endpoint, exposure (frequency, exposure duration, power density), results, and certain quality criteria. Eighty percent of the in vivo studies showed responses to exposure, while 58% of the in vitro studies demonstrated effects. The responses affected all biological endpoints studied. There was no consistent relationship between power density, exposure duration, or frequency, and exposure effects. The available studies do not provide adequate and sufficient information for a meaningful safety assessment, or for the question about non-thermal effects. There is a need for research regarding local heat developments on small surfaces, e.g., skin or the eye, and on any environmental impact. Our quality analysis shows that for future studies to be useful for safety assessment, design and implementation need to be significantly improved.
Keywords: radiofrequency electromagnetic fields, MMW, in vivo, in vitro
1. Introduction
Recent decades have experienced an unparalleled development of technologies that are categorized as information and communication technologies (ICT), which include wireless communication used for mobile telephony (MP) and e.g., Wi-Fi by using electromagnetic fields (EMF). The first generation of handheld mobile phones were available for individual, private, customers in a few countries in the late 1980’s. Subsequently, the second (2G), third (3G), and fourth (4G, LTE) generations increased their penetration rates in the society in a dramatic way, so that today there are more devices than inhabitants of the Earth. In addition, Wi-Fi and other forms of wireless data transfer have become ubiquitous, and are globally available. At present we are starting to introduce the next generation, 5G, of mobile networks. Importantly, 5G is not a new technology, but an evolution of already existing G1 to G4 technologies.
With the upcoming deployment of 5G mobile networks, significantly faster mobile broadband speeds and increasingly extensive mobile data usage will be ensured. This is made possible by the use of additional higher frequency bands. 5G is intended to be the intersection of communications, from virtual reality to autonomous vehicles to the industrial Internet and smart cities. In addition, 5G is considered the base technology for the Internet of Things (IoT), where machines communicate with machines (M2M communication). At the same time, a change in the exposure to electromagnetic fields (EMF) of humans and the environment is expected (see, for example [ 1 , 2 ]).
The 5G networks will work with within several different frequency bands ( Table 1 ), of which the lower frequencies are being proposed for the first phase of the 5G networks. Several of these frequencies (principally below 1 GHz; Ultra-high frequencies, UHF) have actually been or are presently used for earlier mobile communication generations. Furthermore, much higher radio frequencies (RF) are also planned to be used at later stages of technology evolutions. The new bands are well above the UHF ranges, having wavelengths in the centimeter (3–30 GHz) or the millimeter ranges (30–300 GHz; millimeter waves, MMW). These latter bands have traditionally been used for radars and microwave links.
Subdivision of the 5G frequency spectrum.
The introduction of wireless communication devices that operate in the high frequency parts of the electromagnetic spectrum has attracted considerable amounts of studies that focus on health concerns. These studies encompass studies on humans (epidemiology as well as experimental studies), on animals, and on in vitro systems. Summaries and conclusions from such studies are regularly published by both national and international committees containing relevant experts (see e.g., [ 3 , 4 , 5 ]. The conclusions from these agencies and committees are that low level RF exposure does not cause symptoms (“Idiopathic Environmental Intolerance attributed to Electromagnetic Fields”, IEI-EMF), but that a “nocebo” effect (expectation of a negative outcome) can be at hand. Some studies suggest that RF exposure can cause cancer, and thus the International Agency for Research on Cancer classified RF EMF as a “possibly carcinogenic to humans” (Group 2B) [ 3 ]. In a recent recommendation of a periodically working Advisory Group for IARC “to ensure that the Monographs evaluations reflect the current state of scientific evidence relevant to carcinogenicity” the group recommended radiofrequency exposure (among others) for re-evaluation “with high priority” [ 6 ]. There is further no scientific support for that effects on other health parameters occur at exposure levels that are below exposure guideline levels, even though some research groups have published non-carcinogen related findings after RF exposure at such levels (see [ 4 , 5 ]). Environmental aspects of this technological development are much less investigated.
Frequencies in the MMW range are used in applications such as radar, and for some medical uses. Occupational exposure to radars have been investigated in some epidemiological studies, and the overall conclusion is that this exposure does not constitute a health hazard for the exposed personnel [ 7 ]. This is due to that exposures for all practical purposes are below the guideline levels and thus not causing tissue heating. However, further studies are considered necessary concerning the possible cancer risk in exposed workers. Medical use of MMW has been recently reviewed [ 8 , 9 ] suggesting a possibility for certain therapeutic applications, although the action mechanisms are unclear.
The 5G networks and the associated IoT will greatly increase the number of wireless devices compared to the present situation, necessitating a high density of infrastructure. Thus, a much higher mobile data volume per geographic area is to be created. Consequently, it is necessary to build a higher network density because the higher frequencies have shorter ranges. The question that arises, is whether using the higher frequencies can cause health effects?
Exposure limits for both the general public and occupational exposure are available and recommended by the WHO in most countries, based on recommendations from ICNIRP [ 10 ] or IEEE [ 11 ] guidelines. These limits, which have considerable safety factors included, are set so that exposure will not cause thermal damage to the biological material (thermal effects). Thus, for 10 GHz to 300 GHz, 10 W/m 2 is recommended as the basic restriction (no thermal effects), with reference values for 400 MHz to 2 GHz (2–10 W/m 2 ) and >2 GHz (10 W/m 2 ). It should be pointed out that the present ICNIRP guidelines [ 10 ] are currently being revised, and new versions are to be expected in the near future. In addition, ICNIRP proposes two categories of recommendations: (1) the basic restriction values based on proven biological effects from the exposure and (2) the reference levels given for the purpose of comparison with physical value measurements. ICNIRP guidelines present no reference values above 10 GHz, only considering the basic restriction values. This is due to that only surface heating occurs since the penetration depth is so small at these frequencies. Therefore any calculations of the Specific Absorption Rate (SAR) values, that take larger volumes into consideration, are not reasonable to perform.
The SAR is the measure of the absorption of electromagnetic fields in a material and is expressed as power per mass/volume (W/kg), where the penetration depth of the electromagnetic fields depends on the wavelength of the radiation and the type of matter. The penetration depth of MMW is very shallow, hence the exposed surface area and not the volume is considered. The appropriate exposure metric for MMW is therefore the power density, power per area (W/m 2 ).
It is of course too early to forecast the actual exposures to 5G networks. However, the antennas planned for 5G will have narrow antenna beams with direct alignment [ 12 ] to the receiving device. This could possibly significantly reduce environmental exposure compared to the present exposure situation. However, it is also argued that the addition of a very high number of 5G network components will increase the total EMF exposure in the environment, and that higher exposures to the higher frequencies can lead to adverse health effects.
Therefore, the question arises, what do we know so far about the effects on biological structures and on health due to exposure to the higher frequency bands (in this review we consider 6–100 GHz, since lower frequencies have been extensively investigated due to their use in already existing wireless communication networks)? Do so-called “non-thermal” effects (effects that occur below the thermal effect threshold) occur, that can lead to health effects? Is there relevant health-oriented research using the 5G technology relevant frequencies? Is there relevant research that can make a significant contribution to improving the risk assessment of exposure to the general population? Answers to these questions are necessary for a rapid and safe implementation of a technology with great potential.
2. Materials and Methods
This review takes into account scientific studies that used frequencies from 6 GHz to 100 GHz as the source of exposure. The review is based on available data in the field of public literature, papers written in English until the end of 2018 (PubMed database: www.ncbi.nlm.nih.gov/pubmed ), EMF-Portal ( www.emf-portal.org ), and other relevant literature such as documents from ICNIRP, SCENIHR, WHO, IARC, IEEE, etc.). In addition, more refined research was conducted when necessary from sources that were not included in the above-mentioned databases (relevant abstracts from conferences, abstract books, and archives of journals). The resulting studies were examined for technical and scientific data and presented in the supplementary Table S1 .
As a pragmatic approach, we interpreted the results as a “response” when the authors themselves reported the result as an “effect/response” based on a statistical analysis and the p -value < 0.05.
Next we defined necessary criteria for study quality, both from a biomedical and physical point of view (see [ 13 ]). The results of the studies were (if possible) analysed for correlations with study quality according to the correlation approach done by Simkó et al. [ 14 ]. The studies were analysed with reference to a minimum of criteria in terms of experimental design and implementation. The following criteria were considered: were the experiments performed in the presence of an appropriate sham/exposure control, temperature control, positive control, were the samples blinded, and was a comprehensive dosimetry presented.
The study is divided into a descriptive part, which covers the description of all selected studies, their exposure conditions, frequency ranges (6 GHz to 100 GHz), dose levels, etc., as well as the biological results, presented in a Master-Table ( Table S1 ). Review articles were not considered. The outcomes of the studies were furthermore analyzed and discussed according to frequency domains, and power density and exposure duration. If appropriate, we include an evidence-based interpretative part regarding risk from exposures according to the criteria of SCHEER [ 15 ].
In the following, health-related published scientific papers dealing with frequencies from 6 GHz to 100 GHz (using the term MMW for all the frequencies) are described in detail. It should be noted that there are no epidemiological studies dealing with wireless communication for this frequency range, thus, this review will cover studies performed in vivo and in vitro.
Thermal biological effects of radiofrequency electromagnetic fields occur when the SAR values exceed a certain limit, namely 4 W/kg (general population exposure limit: SAR 0.08 W/kg), which causes a tissue heating of 1 °C. However, in the literature, biological effects below 4 W/kg SAR values have been described. Since such effects are considered to be not due to warming, they are termed non-thermal effects. In the present review, in some individual studies, the authors interpreted thermal effects as “no effect”. Those ones and studies without response/effect of MMW exposure were considered as “no response/effect” in our present analysis.
3.1. Grouping of Selected Parameters
For analysis, 94 publications were identified and selected from the accessible databases (in vivo and in vitro) [ 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 ]. It should be noted that the total number of individual examinations is larger than the number of publications, since some authors investigated several physical and/or biological conditions in the same publication.
Various biological endpoints have been identified, which are referred to as “response” or effects when appropriate. Since the list of these endpoints is relatively long, we have not mentioned them in detail, but summarized them in groups: Physiological, neurological, histological changes, or in in vitro studies gene or protein expression, cytotoxic effects, genotoxic changes, and also temperature-related reactions.
For a detailed analysis, a “Master-table” ( Table S1 ) was prepared in which all parameters considered in the studies were included. The table contains the following information: frequency, in vivo or in vitro study (the latter distinguishes between primary cells and cell lines), power density, exposure duration, biological endpoints, and response. Some studies lack information on individual parameters. For example, a publication had to be excluded completely because there was no information about the frequency. In nine studies the power density data were absent and in seven studies the calculated SAR values were provided instead of the power density. In ten studies, the exposure time was not given.
The 45 in vivo studies were mainly conducted on mammals (mouse, rat, rabbit) and a few on humans. In some studies, bacteria, fungi, and other living material were also used for the experiments. 80% of all in vivo studies showed exposure-related reactions.
Primary cells (n = 24) or cell lines (n = 29) were used in the 53 in vitro studies, with approximately 70% of the primary cell studies and 40% of the cell line investigations showing exposure-related responses ( Table 2 ).
Overview of the total number of publications examinations.
All identified studies were analyzed as a function of frequency. For this purpose, frequency domains (groups) have been created ( Figure 1 ) to analyze and illustrate the results. The frequency groups from 30 to 60 GHz were grouped in ten-GHz increments (up to 30, 30.1–40, 40.1–50, 50.1–60 GHz). The frequency range 60–65 GHz was extra analyzed as in this group a larger number of publications was identified (in comparison to the other groups). Due to the low number of publications above 65.0 GHz, data was merged into the groups of “65.1–90” and “above 90 GHz”. As shown in Figure 1 , the majority of studies show a frequency-independent response after MMW exposure.
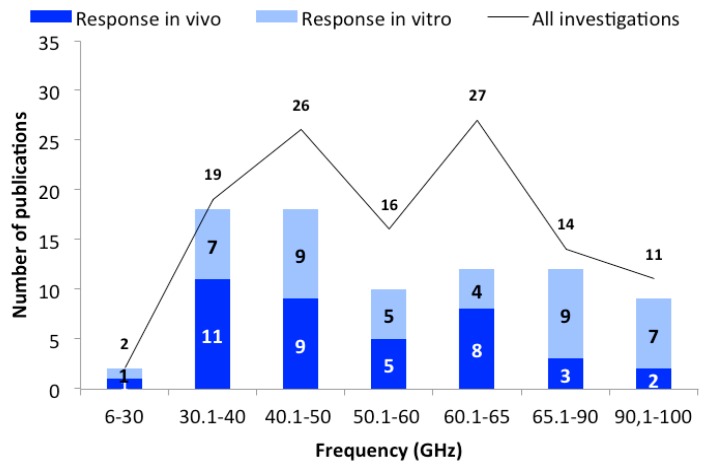
The number of publications as a function of frequency domains. The black line represents the total number of publications, and bars represent the in vivo (dark blue) and in vitro (light blue) studies with biological responses.
3.1.1. Frequency Ranges
All data regarding the individual papers are found in Table S1 .
Up to 30 GHz
The first group “up to 30 GHz” was introduced since some of the 5G frequencies fall within this frequency range. Unfortunately, there are only two publications in this group, both showing responses to the MMW exposure. A study that was conducted on bacteria and fungi showed an increase in cell growth [ 58 ]. The other in vitro study was performed on fibroblasts (25 GHz, 0.80 mW/cm 2 , 20 min), with genotoxic effects observed at high SAR levels (20 W/kg) [ 24 ]. A graphical presentation of the outcomes is presented in Figure 1 for this and all other frequency domains.
Frequency Group 30.1–40 GHz
As shown in Figure 1 , responses were detected in approximately 95% of the 19 studies. In all in vivo studies responses were described after exposure [ 25 , 27 , 36 , 37 , 55 , 56 , 78 , 79 , 87 , 91 , 103 , 104 ]. Endpoints ranged from recorded footpad edema, which is a frequent endpoint for the measurement of inflammatory responses, to morphological changes, changes in skin temperature, blood pressure, heart rate, body temperature, neuronal electrical activity, and EEG analyses. Protein expression studies, oxidative stress marker measurements, histological investigations, and induction of cell death (apoptosis) were performed. Only one study used lower power densities (0.01 mW/cm 2 , 0.1 mW/cm 2 ; SAR: 0.15, 1.5 W/kg; 20 min, 40 min) to study inflammatory responses [ 27 ]. The authors determined the frequency-dependent anti-inflammatory effect as a function of power density and exposure duration and did not rule out temperature-related effects. The power densities of the other in vivo studies were extremely high (10, 75, 500–5000 mW/cm 2 ), so the induced effects were likely temperature dependent.
Eight in vitro studies were performed [ 18 , 20 , 47 , 91 , 97 , 99 , 101 , 102 ] of which seven reported responses. In one study [ 99 ], human blood cells ( ex vivo ) were exposed to MMW for 5, 15 and 30 min (32.9–39.6 GHz, 10 mW/cm 2 ). The activation of the cells was examined in the presence or absence of bacteria. It was shown that in the presence of bacterial activation and after 15 min of exposure, the cells were activated to release free radicals. These results were similar to the heated samples (positive controls), so a temperature effect is plausible. The induction of differentiation of bone marrow cells in to neuronal phenotype cells was also demonstrated (36.11 GHz, 10 mW/cm 2 , 3 × 10 min every 2 h for 24 h) [ 97 ]. In two studies, temperature-related reactions were described at the protein level [ 18 , 91 ]. When the cell cultures were cooled during exposure to prevent the induced temperature increase, no responses were detected.
In three publications, a research group described cell cycle changes, induction of cell death and activation of differentiation processes in primary cells (rat bone cells and mesenchymal stem cells) after exposure to 30–40 GHz (4 mW/cm 2 , different exposure durations) [ 47 , 101 , 102 ]. Unfortunately, the minimum quality criteria were not fulfilled in any of the three studies, mainly because there were no temperature controls.
Frequency Group 40.1–50 GHz
In the 40.1–50 GHz frequency group, 26 studies were identified, 13 in vivo [ 16 , 17 , 26 , 48 , 49 , 51 , 53 , 65 , 69 , 74 , 80 , 84 , 98 ] and 13 in vitro [ 29 , 30 , 31 , 62 , 64 , 86 , 89 , 92 , 93 , 100 , 105 , 107 ] with nine studies showing responses. A large number of studies have tested cell biology endpoints such as cell proliferation, gene or protein expression, and changes in oxidative stress. In addition, immunological, neurological, morphological and genotoxic effects were investigated. The power densities used vary enormously, from 0.02 to 450 mW/cm 2 , and one publication gave no information.
In healthy volunteers, a double-blind study was performed to investigate the effects of MMW on experimentally induced cold pain (42.25 GHz, <17.2 mW/cm 2 , 30 min) [ 74 ]. The authors found no difference from the placebo effect. This study was a repeat of a previous study with volunteers and the results of the older study could not be confirmed. The other four in vivo studies with no detectable effects were investigating genotoxic effects or oxidative stress [ 17 , 48 , 49 , 98 ].
Five in vivo publications addressed the effects of MMW on the immune system of mice or rats, finding activation of the immune system at both the cellular and molecular levels (41.95 or 42.2 GHz, 19.5 μW/cm 2 , 0, 1, 31.5 mW/cm 2 , 20 min or intermittently over 3 days) [ 26 , 48 , 51 , 53 , 84 ].
MMW exposure of frog isolated nerve cells, (41.34 GHz, 0.02, 0.1, 0.5, 2.6 mW/cm 2 , 10–23 min) lead to a reduction of the action potential frequency. Interestingly, the effects at higher power density (2.6 mW/cm 2 ) were similar to conventional heating [ 49 ].
One study detected an increase in the motility of human spermatozoa after 15 min of exposure (42.25 GHz, 0.03 mW/cm 2 ) [ 100 ]. Additional in vitro tests have identified the formation of free radicals, the activation of calcium-dependent potassium ion channels (around 42 GHz, 100, 150, 240 μW/cm 2 , 20–40 min) as well as changes at the cell membrane in exposed cells [ 29 , 30 , 100 ].
No responses on cell biological endpoints (cell cycle changes, cell death, heat shock proteins) were detected in four additional in vitro studies.
Frequency Group 50.1–60 GHz
We identified 16 studies in the frequency group 50.1-60 GHz (six in vivo, ten in vitro) and 60% of the studies showed responses to MMW exposures [ 21 , 23 , 35 , 38 , 43 , 46 , 59 , 61 , 72 , 77 , 81 , 83 , 85 , 94 , 109 ].
In five of the in vivo studies very different responses were shown. In a study on healthy volunteers, the authors wanted to find out whether the human skin at a so-called acupuncture point has different dielectric properties during exposure to MMW. They found that these properties change during exposure to 50–61 GHz from the surrounding skin [ 23 ].
A pilot study on mice (60 GHz, 0.5 mW/cm 2 , lifelong exposure for 30 min/5 days a week) showed that MMW exposure affects cancer-induced cells and increases in motor activity of healthy mice [ 61 ].
In rats, the influence of 54 GHz, 150 mW/cm 2 , on an area of approximately 2 cm 2 on the head was examined [ 81 ]. This transcranial electromagnetic brain stimulation induced pain prevention and prevented the conditioned avoidance response to a pain stimulus in 50% of the animals. However, no changes were detected when serotonin inhibitors were previously administered. Therefore, the authors concluded that transcranial electromagnetic brain stimulation promotes the synthesis of serotonin, a transmitter that changes the animals’ pain threshold.
The effects of MMW were also tested (60 GHz, 475 mW/cm 2 , 1.898 mW/cm 2 , 6, 30 min) on rabbit eyes, describing acute thermal injuries of various types [ 38 ]. The authors also pointed out that the higher temperature just below the eye surface could induce injury.
Neurological investigations were performed on leeches (60 GHz, 1 min, 1, 2, 4 mW/cm 2 ) [ 77 ] and electrophysiological studies were performed on frog oocytes (60 GHz, up to 5 min) [ 85 ]. In both experimental systems effects were described, which were induced by the temperature rise.
Cell biological and morphological changes after exposure to 0.7–1.0 μW/cm 2 (intermittent) were reported in three in vitro studies [ 72 , 83 , 94 ], with two publications providing no information regarding power density or exposure duration. At the level of protein analysis and total genome analysis no changes were identified in four in vitro studies [ 35 , 46 , 59 , 109 ].
Frequency Group 60.1–65 GHz
The number of studies in the 60.1–65 GHz frequency group is 27. Of these, twelve reported effects from exposure to MMW, and no responses were found in 15 studies.
The in vivo studies investigated different topics [ 23 , 27 , 44 , 52 , 67 , 68 , 70 , 71 , 73 , 75 , 76 ]. Thus, two studies examined the effects on tumor development in mice injected with tumor cells [ 52 , 70 ]. In one of the studies it was reported that exposure to 61.22 GHz, 13.3 mW/cm 2 , inhibited the growth of melanoma cells (exposure 15 days after tumor cell injection, 15 min/day) [ 70 ].
Other publications from one research group investigated the potential of MMW for pain relief and the associated biological mechanisms of action [ 67 , 71 , 73 , 75 , 76 ]. Several of the studies were performed on mice skin exposed to 61.22 GHz for 15 min. The most commonly used power density was 15 mW/cm 2 . Another study addressed the dose issue with no effect below 1.5 mW/cm 2 . The authors’ conclusion is that MMW can lower the hypoalgesia threshold, which is likely mediated by the release of opioids.
The effects of 61.22 GHz exposure of mice were examined also with respect to the immune system [ 52 ]. The animals were exposed on three consecutive days for 30 min per day. The exposure caused peak SAR values of 885 W/kg on the nose of the animals where the exposure took place. The power density was 31 mW/cm 2 and the measured temperature rise reached 1 °C. It was found that MMW modulates the effects of the cancer drug cyclophosamide. In particular, the T-cell system of the immune system was activated and various other immune system relevant parameters affected.
The similar exposure condition was used in a study on gastrointestinal function, however no effects were identified [ 68 ].
A single exposure for eight hours (61 GHz, 10 mW/cm 2 ), or five times four hours, did not cause eye damage to rabbits and rhesus monkeys [ 44 ]. It should be emphasized that several of the mentioned studies come from the same laboratory, and all criteria for the study quality are met. However, the authors were able to replicate their own findings on pain relief whereas other laboratories have not replicated this work. In the in vitro studies, various biological endpoints were examined [ 28 , 32 , 33 , 34 , 42 , 45 , 50 , 59 , 60 , 66 , 83 , 88 , 94 , 95 , 108 ].
In one study, neurons of snails ( Lymnea ) were exposed at 60.22–62.22 GHz and no non-thermal responses on the ion currents were identified [ 28 ].
In a series of investigations with nerve cell-relevant cell lines, the dopamine transmission properties, stress, pain and membrane protein expression were investigated (60.4 GHz, 10 mW/cm 2 , 24 h) and no responses were detected [ 32 , 33 , 34 , 59 , 60 , 108 ].
The same exposure setup has also been used in studies examining different stress response related genes (0.14–20 mW/cm 2 ) [ 59 ]. No effects were found at the gene expression level. Interestingly, the overall genome impact was influenced when the exposure (60.4 GHz, 20 mW/cm 2 , 3 h) of the primary human keratinocytes was combined with 2-deoxyglucose, a glucose-6- phosphatase inhibitor. This co-exposure caused a change in the amount of six different transcription factors, the effect differing from the effect of 2-deoxyglucose alone and 60.4 GHz alone (both factors alone induced no changes).
Other studies also examined human keratinocytes and astrocytoma glial cells after exposure to 60 GHz (0.54, 1 and 5.4 mW/cm 2 ) [ 60 , 108 ]. Various parameters such as cell survival, intracellular protein homeostasis, and stress-sensitive gene expression were investigated. Also, in these studies, no effects were observed. In contrast, in one publication, the elevation of an inflammatory marker (IL1-β) was observed in human keratinocytes after exposure (61.2 GHz, 29 mW/cm 2 , 15, 30 min), while other inflammatory markers (chemotaxis, adhesion and proliferation) have remained unchanged [ 95 ].
Another type of study was performed on rat brain cortical slices [ 66 ]. The brain slices were exposed to a field of 60.125 GHz (1 μW/cm 2 ) for 1 min, and then specific electrophysiological parameters were measured. In many slices, transient responses on membrane characteristics and action potential amplitude and duration were observed. The exposure caused a temperature rise of the medium (of 3 °C) in which the sections were stored. Interestingly, a chronically induced Ca 2+ blockade did not affect the MMW response.
Frequency Group 65.1–90 GHz
The studies in the frequency group of 65.1 to 90 GHz were performed both in vivo and in vitro in a total of 14 articles (four in vivo and 11 in vitro investigations). The studies vary widely, based on different hypotheses, biological endpoints, power densities, and exposure durations. In addition, some studies have used biological materials to identify physical properties such as dielectric properties and skin reflection coefficient. The latter studies are discussed in Section 4.2 .
Four in vivo studies reported responses after MMW exposure. One study examined the dose of eye damage (especially damage to the corneal epithelium) [ 40 ]. The dose was calculated as DD 50 (based on the results for which the probability of eye damage was 50%). The experiments were carried out on rats with an exposure of 75 GHz, the DD 50 value being 143 mW/cm 2 .
Other in vivo studies were performed on rats and mice as well as on insects [ 27 , 42 , 57 ]. The study on mice used different frequencies of 37.5 to 70 GHz, with power densities of 0.01 and 0.3 mW/cm 2 for 20 to 40 min. A single whole-body exposure of the animals reduced both the footpad edema and local hyperthermia on average by 20% at the frequencies of 42.2, 51.8, and 65 GHz. Other frequencies had no influence.
The study on insects ( Chironomidae ) focused on DNA effects of giant chromosomes of the salivary glands of the animals with different frequencies (64.1–69.1, 67.2, 68.2 GHz) [ 42 ]. All frequencies, using power densities <6 mW/cm 2 , caused a reduction in the size of a particular area of the chromosome. This in turn led to the expression of certain secretory proteins of the salivary gland.
Different aspects were studied in the in vitro studies [ 18 , 28 , 39 , 50 , 64 , 72 , 83 , 89 , 94 , 106 ], where nerve cell function was investigated in three studies. Two studies used nerve cells from the snail Lymnea that were exposed at 75 GHz for a few minutes at very high SAR levels (up to 4200 W/kg, power density was not reported) [ 28 , 39 ]. The authors observed thermal effects on the ion currents and the firing rate of the action potentials. Another study also described thermal effects on transmembrane currents and ionic conductivity of the cell membrane. Again, the exposure was at very high SAR levels (2000 W/kg), and the authors emphasized the temperature dependence of the reaction.
Broadband frequencies (52–78 GHz) have been used in several publications, mainly investigating the effects on cell growth and cell morphology as well as the ultrastructure of different cell lines [ 50 , 72 , 83 , 94 ]. The values for the power densities were not given consistently but appear to have been very low (not higher than 1 μW/cm 2 ). The results indicated the inhibition of cell growth, accompanied by changes in cell morphology.
Another group of studies used hamster fibroblasts, BHK cells, and exposed the cells at 65 to 75 GHz, with the power density reaching 450 mW/cm 2 [ 18 , 64 , 89 ]. The authors noted the inhibition of protein synthesis and cell proliferation as well as cell death at higher power densities. In a study using human dermal fibroblasts and human glioblastoma cells, no effects at the protein level (proliferation or cytotoxicity markers) were detected (70 GHz and higher, in 1 GHz increments; 3, 70 or 94 h) [ 106 ]. Power densities varied across frequencies, ranging from 1.27 μW/cm 2 in the lower frequency range to 0.38 μW/cm 2 at higher frequencies.
The in vitro studies in this group are similar to the in vivo studies in their diversity. The majority of studies in which responses were reported are thermal-effects due to MMW exposure. In three studies, responses at low power densities were described, but all results were from the same laboratory, and were not replicated by others. Moreover, the quality of these studies is questionable, as the quality criteria were not met.
Frequency Group 90.1–100 GHz
Eight out of eleven studies in the 90.1–100 GHz frequency group are in vitro studies [ 22 , 41 , 57 , 82 , 90 , 96 , 106 ]. The three in vivo investigations addressed a variety of issues including acute effects on muscle contraction, skin-reflection properties (which are more of a dose-related than health-related issue), and skin cancer [ 19 , 54 , 57 ]. The rat skin cancer study (one to two weekly, short-term exposures at 94 GHz, 1 W/kg; DMBA-initiated animals) did not show any positive outcome [ 54 ]. Another study examined the muscle contraction of mice and described some responses [ 19 ]. Again, 94 GHz was used, but power density or SAR values were not reported.
Seven of the eight in vitro studies showed responses after MMW exposure. In some studies, primary neurons were used to study the cytoskeleton (94 GHz, 31 mW/cm 2 ) [ 82 ] or specific electrophysiological parameters (90–160 GHz) [ 22 ]. In the latter study it was found that the observed responses were more likely due to interactions with the cell culture medium than with the cells, although the mechanisms of action were not clear. Other studies identified responses on the DNA integrity (100 GHz and higher) [ 41 ] or described changes in intracellular signaling pathways (94 GHz, 90–160 GHz) using different cell types [ 57 , 96 ]. The exposure time ranged from minutes to 24 h for partially unknown exposure values. In one study no cytotoxic influence at power density levels of a few μW/cm 2 was detected in either normal or in tumor cells.
3.1.2. Power Densities
All identified studies were analyzed as a function of the used power densities. The studies were grouped depending on the power density as follows: below 1; 1.1–10; 10.1 to 50; 50.1–100, and 100.1 mW/cm 2 or higher. Studies that do not provide information on power density or SAR values are not displayed in these groups. As shown in Figure 2 , the vast majority of studies show responses regardless of the power density used.
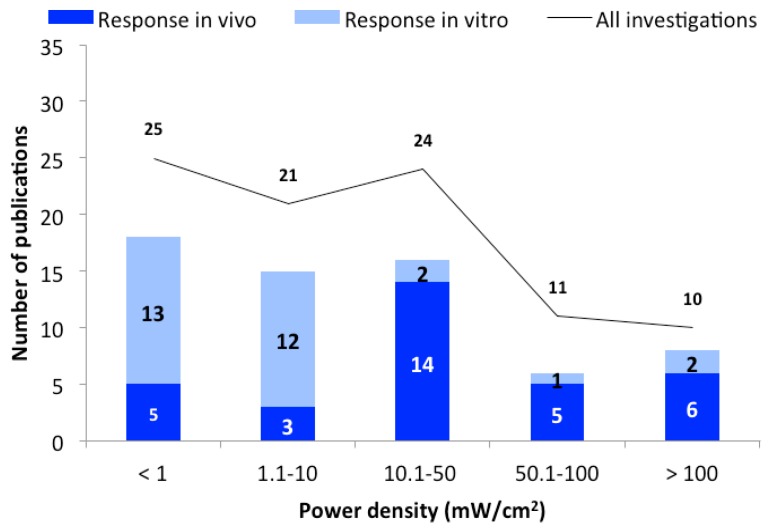
The number of publications as a function of power density. The black line represent the total number of publications, and bars represent the in vivo (dark blue) and in vitro (light blue) studies with biological responses.
3.1.3. Exposure Duration
Exposure duration of the studies was also grouped for data analysis ( Figure 3 ). The time groups were selected as seconds to 10 min; 10–30 min; 30–60 min; over 60 min-days and alternately/intermittently. The groups were selected so that the used exposure times and the number of studies are meaningfully summarized. Here, too, it becomes clear that the majority of all studies show responses regardless of the exposure time. Interestingly, longer exposure times (over 60 min—days) seemingly lead to fewer reactions than in the other groups.
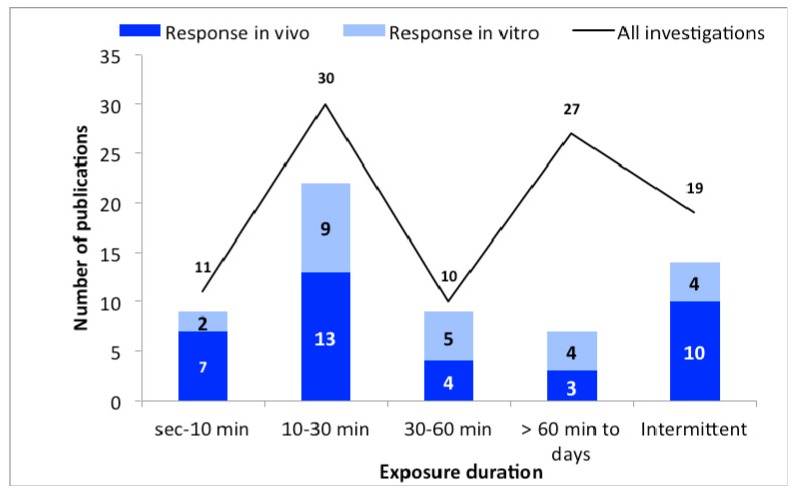
The number of publications as a function of exposure duration. The black line represent the total number of publications, and bars represent the in vivo (dark blue) and in vitro (light blue) studies with biological responses.
3.2. Studies without Responses
Table 3 shows the number of studies in which no responses were detected after or during MMW exposure. As “no response” also such investigations were referred to, which were considered by the authors themselves as such. This means that in some cases the observed effects were described as temperature-related and not as a non-thermal MMW effect.
Studies without responses.
Few in vivo studies have shown no response at all. Noticeable is the frequency group 40.1–50 GHz, in which 6 studies were identified. These studies investigated immunosuppression, genotoxic effects, changes in pain sensitivity, and changes in enzyme activity. One study was carried out on bacteria and fungi.
There are a variety of in vitro studies in which no responses were detected. Interestingly, studies on protein or gene expression levels often failed to detect any changes after MMW exposure. This could be due to the fact that in in vitro studies the possibility of non-thermal effects were specifically investigated, where cooling was used to counteract the temperature increase.
3.3. Quality Analysis
We analyzed the quality of the selected studies according to specific criteria [ 14 ]. The studies were categorized by the presence of sham/control, dosimetry, positive control, temperature control, and whether the study was blinded. The presence of these five criteria while performing an MMW study is the minimum requirement for qualifying as a study with sufficient technical quality.
Of the 45 in vivo studies, 78% (35) demonstrated biological responses after exposure to MMW. Of all studies, 73% were performed with sham/controls, 76% employed appropriate dosimetry, 44% used positive control, and 67% were done under temperature control conditions ( Figure 4 ). Unfortunately, only 16% of the studies were performed according to protocols that ensured blinding and only three publications were identified that met all five criteria [ 26 , 51 , 53 ]. If the blinding criterion was excluded, 13 studies could be identified that met the remaining four criteria. Considering three criteria only, namely sham, dosimetry, and temperature control, 40% (20 papers) were identified. Thus, the quality of the in vivo studies is unsatisfactory.
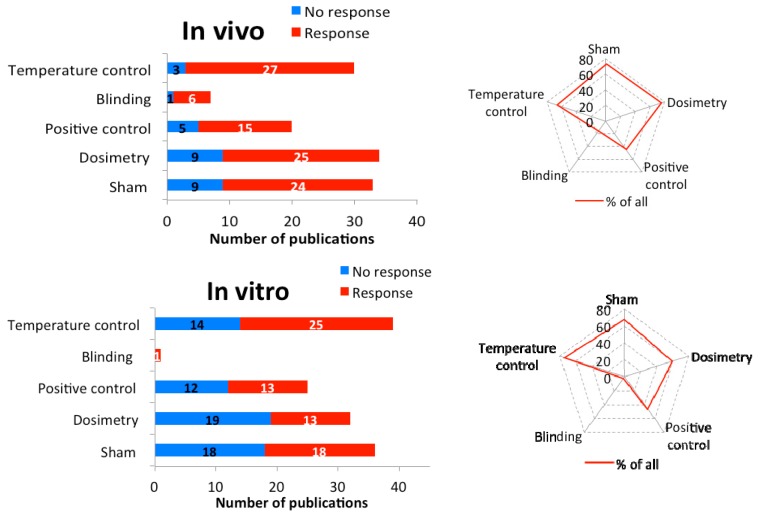
The quality of all publications: The number of in vivo (top) and in vitro (bottom) experiments (blue: no reaction, red: reaction) using the listed quality features (y-axis). The spider web shows the percentage of the quality characteristics in all examinations.
Out of the 53 in vitro studies, 31 showed biological responses. Only in 13 studies (42%) were three of the five quality criteria satisfied, namely the presence of sham/control, dosimetry, and temperature control ( Figure 4 ). Positive controls were used in 47% and only one study was performed with blinded protocol (2%).
These results show that the number of examinations and the quality criteria are insufficient for a statistical analysis. It should be stressed that this quality analysis covers all publications dealing with the responses/effects of exposure to 6 to 100 GHz MMW, irrespective of the endpoints tested. To perform a correlation analysis, a larger number of comparable studies (e.g., identical endpoints in a frequency group) would be required.
4. Discussion
The first relevant observation during the analysis of the studies is that in most publications the aim of the investigations has been to determine the effects of MMW exposure for medical purposes. This means that the exposure devices used primarily come from medical applications (therapy or diagnostics). Very few publications dealt with health-related issues after MMW exposure in general, or with the specific topic of 5G. Therefore, the 94 publications are very heterogeneous.
We divided the frequency bands into seven ranges and placed the studies in the relevant groups. All available information on physical and experimental parameters was collected, but the exact number of experiments in each study was not taken into account. (One publication can contain more than one experiment.) Therefore, it is the provided numbers of studies/publications that constitute the data set, not the exact numbers of experiments performed, which is significantly higher.
This report does not provide a statistical analysis of the correlation between the exposure conditions and the results, which was our original ambition. In the correlation study according to Simkó et al. [ 14 ] a frequency group was selected, with only one group of biological endpoints considered. About one hundred, exclusively in vitro, studies were identified and broken down into individual experiments in that paper. In this way, the number of experiments was sufficient to perform a correlation analysis. In the present review, the spread of biological endpoints in the individual frequency groups and the models used (in vivo and in vitro) is large and the number of studies is very low. Therefore, it was not possible to group the studies by specific endpoints and perform a statistical analysis.
Interestingly, more than half of the studies (53 publications) were conducted in the frequency bands 40.1–50 and 60.1–65 GHz (with different models and endpoints). One possible reason for this is that medical use of MMW has a long tradition in Eastern Europe. These applications use specific frequencies that fall in these two frequency groups. The studies were conducted with the aim of testing specific effects with medical relevance. In these two frequency groups, the “with responses” percentage was generally lower than in the other frequency bands (see Figure 1 ), where a majority of studies showed responses to exposure.
With regard to the power densities used, about half of the studies were carried out in the range up to 10 mW/cm 2 ( Figure 2 ). This value is ten times higher than the current ICNIRP exposure guideline [ 10 ] for the general population. Based on available data, there is no indication that higher power densities cause more frequent responses, since the percentage of responses in all groups is already at 70% ( Figure 2 ). One exception from this high response rate is the group 50.1–100 mW/cm 2 , where the proportion of studies with reactions is slightly lower (54%). However, the total number of examinations (11) is relatively small in this group.
The results of some of the studies may suggest that exposure to power densities at or below the guideline recommendations induce biological effects. There are, however, some arguments against it. One of these is the apparent heterogeneity of the study design and the outcomes studied. There are very few (if any) independent replication studies that confirm the reported results. It is also noteworthy that there is no trend towards a classic dose-response pattern where stronger or more frequent effects would be caused by higher exposure levels. Since the studies with conditions promoting tissue warming show no greater effect than below the guideline values (1 mW/cm 2 ), this would either mean that the same interactions are present at all power densities tested, or that experimental artifacts unknown to the scientists are present.
The most important physical experimental parameter is the temperature during exposure, therefore, the temperature must be consistently controlled. The need for stringent temperature control is not an insignificant or trivial matter and has been neglected or at least undervalued in many studies. Although some authors report that they performed specific temperature measurements during the experiments, this does not necessarily mean that this represents the actual temperature in the biological material. Measurements can be made, for example, in the surrounding medium but not in the exposed tissue or in the cell. It also has to be considered that the “bulk” heating (from outside to inside with a certain time course) can differ from a heating that occurs at a rather limited point (“hot spot”). In addition, the intensity of a short burst can be lost if the measurements are based on average exposure times. Such errors and problems are possible factors that have contributed to the questionable interpretation of “non-thermal effects” in some studies.
Effects after MMW exposure were shown at all exposure times with no clear time dependency. The data presented shows one exception, namely in the group “>60 min to days”, where fewer reactions were detected ( Figure 3 ). It has to be taken into account that 27 examinations were carried out in this group, 23 of which were in vitro studies. In vitro experiments can be carried out under cooling, therefore the results can be different (see further below).
Two research groups together provide 30 of the 94 publications in the data set, and could thus possibly have a large impact on the analysis of the outcomes. One group presented at least 21 publications (42.25 and 61.82 GHz; 10 to 30 mW/cm 2 ; with different exposure durations), with a variety of in vivo and in vitro studies, which mostly reported responses to exposure. The other group mainly studied gene and protein expressions (60 GHz; 5.4 to 20 mW/cm 2 ; exposure durations from minutes to days) and found mainly no responses. Studies from both groups adhered well to the quality criteria in our analysis.
4.1. Temperature Controls in In Vitro Studies
In vivo studies that are performed within or directly on the living organism have shown both thermal and purportedly non-thermal effects after or during MMW exposure. In vitro studies are carried out on cells and most experimental parameters can be accurately set and observed. Cell cultures can thus be very carefully controlled, e.g., an induced temperature increase can be counter-cooled. Many in vitro studies considered in this review were performed using cooling of the cell culture vessels and the authors did not detect any non-thermal effects in these studies. In in vivo studies counter-cooling is not possible, thus it is very difficult to differentiate between thermal and non-thermal reactions. Therefore, in vivo and in vitro studies regarding the induced effects cannot be directly compared. An accurate dosimetry could solve this problem.
4.2. Dosimetry
It is important to know what the exposure of the MMW will be due to the expected introduction of a large number of 5G wireless communication devices. Given the novelty of the technology, it is currently unlikely that a large number of relevant exposure assessment studies will be available. However, an example from a recent study [ 110 ] shows that a “typical” office environment with wireless communication transmitters (5.50 GHz) leads to power densities well below the exposure guideline limits. Thus, the maximum power density was measured at 0.89 μW/cm 2 .
Partly (n = 25) the experimental studies on biological and health effects of MMW exposure are at or below the ICNIRP exposure guidelines. The power densities were often chosen so that the exposure caused no or very moderate tissue warming (<1 °C), namely in the range of 1 to 10 mW/cm 2 . Since the penetration into the tissue of these frequencies are on the order of millimeters and below, it is important to study biological effects directly or indirectly related to skin and eyes exposure. As mentioned previously, the number of available studies in the 6–100 GHz frequency range is relatively low, which is in contrast to the number of studies for lower radio frequencies. Similarly, the number of tissue dosimetry studies (especially for the skin) is very limited. However, such studies are very relevant because they show how certain exposure parameters can influence the energy input and thus the thermal behavior of the skin.
Currently, both the ICNIRP guidelines and the IEEE standards are being revised to replace the SAR values with power density above 6 GHz. However, it has already been recognized that there is a reactive near field close to the transmitter (around the antennas). Here, the energy is not radiated, but the energy envelopes the antennas. The question is whether these “reactive near fields” are important for the energy delivery to a human body near the transmitter? If this is not the case, it is sufficient to comply with the existing exposure limits based on free space power density measurements. On the other hand, a strong reactive near field would considerably complicate the exposure situation [ 111 ]. Therefore, for dosimetry modeling of distances (from the antenna) below the wavelength of the MMW (mm), temperature measurements should rather be performed in suitable phantoms rather than direct measurements of the power densities in the free space [ 111 ].
The question is how reliably the power density (in free space) can be extrapolated to possible temperature increases in human tissue? For example, Neufeld et al. [ 112 ] found that 10 GHz “bursts” (considered “safe” by ICNIRP and IEEE) can cause temperature increases of >1 °C if the burst duration is long enough. It was also discussed whether the average values of the power densities for the safety assessment are the right ones. In addition, the temperature increase by the MMW also depends on the size of the area. Thus, the factors such as the amplitude of the burst, the “averaging area” and the “averaging time” for the dosimetry would have to be considered.
Foster et al. [ 113 ] reviewed and modelled data on MMW-induced temperature increases in human skin. The model takes into account the frequencies of 3–100 GHz and smaller skin areas with the diameter of 1–2 cm. Available data on exposures lasting more than a few minutes, as well as areas of skin larger than 2 cm in diameter, were limited and made modeling difficult, but consistent with existing data. This means that this model, after appropriate evaluation for dosimetry, could use smaller areas of the skin. The authors also commented on the exposure guidelines for frequencies from 3 to 300 GHz in a separate article [ 114 ]. Based on “thermal modeling,” the authors considered the current guidelines to be conservative in terms of protection against temperature increases in the tissue. They also pointed out that the averaging time and average area provisions require further refinement and that the effects of short high intensity bursts may not be protected by the guidelines.
Zhadobov et al. [ 115 ] addressed the problem of accurate temperature measurement in in vitro MMW studies. They found that the type of thermal probe (thermocouples are better than fiber optic probes) and the size of the probe (smaller probes are more accurate) are relevant. In addition, they were able to show that the initial temperature rise during exposure is rapid (within seconds until a plateau is reached) and that the cells absorb very small amounts of energy, since most of the energy is already absorbed in the cell culture medium. Nevertheless, the authors have calculated that the exposure of 58.4 GHz with 10 mW/cm 2 leads to SAR values of more than 100 W/kg in a cell monolayer. This value is a fraction of the SAR values of the fluid surrounding the cells.
Several studies focused on the distribution of power density and the change in skin temperature as a result of exposure to MMW in the 6 to 100 GHz frequency range. The studies are experimental and/or modeling studies using previously published data. Alekseev et al. [ 116 , 117 ] investigated the absorption of the skin of mice and humans at frequencies between 30 and 82 GHz (10 mW/cm 2 ). They found that in both species absorption into both the epidermis and the dermis occurs with a concomitant loss of power density in the deeper regions. An extended study from the same group [ 118 ] on human forearm skin showed that both temperature increase and SAR values depend on frequency (in the interval of 25 to 75 GHz; 25, 73.3 and 128 mW/cm 2 ).
Frequency dependence for temperature increases was also observed in a modeling study with human facial skin [ 119 ]. Pulsed MMWs were used (6–100 GHz, 100 mW/cm 2 , 200–10,000 ms pulse length) and the skin temperatures were modeled as the function of both pulse length and frequency. Peak skin temperature increased as a function of frequency up to 20 GHz, while above 20 GHz it proved to be dependent on “absorption hotspots”. In deeper regions (>2 mm), the temperature increases were very low and highest around 10 GHz.
In addition, certain skin constituents have been shown to affect energy absorption. It has been shown that the presence of sweat glands [ 120 , 121 ] and also capillaries in the dermis can cause locally elevated SAR levels [ 122 ]. The latter study showed that SAR levels in vessels could be up to 30 times higher than in the surrounding skin, depending on the diameter of the vessels.
Both [ 23 ] and [ 123 ] have reported that the dielectric properties of different areas of the skin differ. The first study found that so-called acupuncture points in healthy volunteers show different dielectric properties when exposed to MMW (50–75 GHz, 14 mW/cm 2 ), while the second study even found differences between the epidermis and dermis (0–110 GHz).
These studies suggest that both the frequency and the specific condition and composition of the skin are relevant for tissue dosimetry. However, too few and very different studies are available to give a conclusive picture on dosimetry of 5G-relevant MMW exposures.
4.3. ICNIRP and other Exposure Recommendations
The guidelines for exposure limits for radiofrequency electromagnetic fields from 3 to 300 GHz in many countries are based on the recommendations of the International Commission on Non-Ionizing Radiation Protection (ICNIRP) [ 10 ]. However, there are also other organizations dealing with limit values such as the Institute of Electrical and Electronics Engineers, IEEE [ 11 ] or the US Federal Communications Commission, FCC [ 124 ].
The guidelines contain basic exposure limits that are indicated as SAR or power density. The limits for a given frequency differ only slightly, if at all, between the different guidelines. However, an important difference between the guidelines concerns frequency, as the SAR basic restriction values change to power density. This frequency (range) is currently set by ICNIRP at 10 GHz, while IEEE and FCC see this between 3–6 GHz. The current revision of these guidelines aims to harmonize these frequencies.
The exposure limits specified in the guidelines should protect against warming of tissue above 1 °C. The reason is that the perceived dangers of MMW energy are associated with excessive heating, called thermal effects. However, it must be considered that the guidelines mean a temperature increase of 1 °C relative to the starting temperature, regardless of the starting temperature. Elevations in temperature may cause pain in the skin when moderately increased, whereas at temperatures of 43–44 °C it may even induce burns [ 124 , 125 ].
At present, only thermal effects due to high-frequency electromagnetic fields are recognized as effects. This means that effects have a thermal component even if it is obviously not due to tissue that has been damaged by excessive heating. On the other hand, it has been suggested that the MMW exposure may also cause non-thermal effects. So far, however, no recognized expert committee has supported such an assertion.
4.4. Knowledge Gaps and Research Recommendations
Exposure of humans can occur through 5G devices with frequencies above 6 GHz, and may be primarily on the skin and, to a lesser extent, on the eyes. This is due to the very low penetration depth of this MMW. Therefore, it is important to investigate whether there are any health-related effects on the skin and/or effects associated with the skin. These include acute skin damage from tissue heating (burns), but possibly also less acute effects (such as inflammation, tumor development, etc.). Such effects could appear after prolonged and repeated heating of superficial structures (the skin). This would mean that thermal effects occur that are not due to acute but to chronic damage.
It may also be that local exposure causes energy deposition in the dermis of the skin, which may be so great as to affect nerve endings and peripheral blood vessels through warming mechanisms. Such scenarios were proposed by Ziskin [ 9 ] based on a series of studies by his group. These studies typically used exposures around 60 GHz at a power density of 10 mW/cm 2 on the skin in the sternum area to produce systemic effects. The aim was to treat certain diseases and complaints. The idea was that the treatment induces the release of the body’s own opioids and additionally stimulates the peripheral nerves. The stimulation would depend on a local thermal effect, which, due to the frequencies, induces locally high SAR values, even at low power densities, thus warming the tissue.
Due to the contradictory information from various lines of evidence that cannot be scientifically explained, and given the large gaps in knowledge regarding the health impact of MMW in the 6–100 GHz frequency range at relevant power densities for 5G, research is needed at many levels. It is important to define exact frequency ranges and power densities for possible research projects. There is an urgent need for research in the areas of dosimetry, in vivo dose-response studies and the question of non-thermal effects. It is therefore recommended that the following knowledge gaps should be closed by appropriate research (the list of research recommendations is not prioritized):
Exact dosimetry with consideration of the skin for relevant frequency ranges, including the consideration of short intense pulses (bursts)
Studies on inflammatory reactions starting from the skin and the associated tissues
In vivo studies on the influence of a possible tissue temperature increase (e.g., nude mouse or hairless mouse model)
In vivo dose-response studies of heat development
Use of in vitro models (3D models) of the skin for molecular and cellular endpoints
Clarification of the question about non-thermal effects (in vitro)
There are also questions about the environmental impact, with potential consequences for human health. Since many MMW devices will be installed in the environment, the impact of MMW on insects, plants, bacteria, and fungi is relevant to investigate. Particularly relevant is the question of temperature increase in very small organisms, as the depth of penetration of the MMW could warm the whole organism.
An unrealistic scenario, however, is that MMW exposures at realistic power densities could cause systemic body warming in humans. Any local heat exposure would be dissipated by the body’s normal heat regulation system. This is mainly due to convection caused by blood flow adjacent to the superficial skin areas where the actual exposure takes place.
In summary, it should be noted that there are knowledge gaps with respect to local heat developments on small living surfaces, e.g., on the skin or on the eye, which can lead to specific health effects. In addition, the question of any possibility of non-thermal effects needs to be answered.
5. Conclusions
Since the ranges up to 30 GHz and over 90 GHz are sparingly represented, this review mainly covers studies done in the frequency range from 30.1 to 65 GHz.
In summary, the majority of studies with MMW exposures show biological responses. From this observation, however, no in-depth conclusions can be drawn regarding the biological and health effects of MMW exposures in the 6–100 GHz frequency range. The studies are very different and the total number of studies is surprisingly low. The reactions occur both in vivo and in vitro and affect all biological endpoints studied.
There does not seem to be a consistent relationship between intensity (power density), exposure time, or frequency, and the effects of exposure. On the contrary, and strikingly, higher power densities do not cause more frequent responses, since the percentage of responses in most frequency groups is already at 70%. Some authors refer to their study results as having “non-thermal” causes, but few have applied appropriate temperature controls. The question therefore remains whether warming is the main cause of any observed MMW effects?
In order to evaluate and summarize the 6–100 GHz data in this review, we draw the following conclusions:
Regarding the health effects of MMW in the 6–100 GHz frequency range at power densities not exceeding the exposure guidelines the studies provide no clear evidence, due to contradictory information from the in vivo and in vitro investigations.
Regarding the possibility of “non-thermal” effects, the available studies provide no clear explanation of any mode of action of observed effects.
Regarding the quality of the presented studies, too few studies fulfill the minimal quality criteria to allow any further conclusions.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/16/18/3406/s1 , Table S1: Master-table of the selected (in vivo and in vitro) studies and the extracted physical, biological, and quality parameters.
Author Contributions
M.S. and M.-O.M. have contributed equally to conceptualization, structuring, data collection and analysis, interpretation of data, and all aspects of writing of the manuscript.
This research was funded by Deutsche Telekom Technik GmbH, Bonn, Germany, PO number 4806344812.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
- 1. Chávez-Santiago R., Szydełko M., Kliks A., Foukalas F., Haddad Y., Nolan K.E., Kelly M.Y., Masonta M.T., Balasingham I. 5G: The Convergence of Wireless Communications. Wirel. Pers. Commun. 2015;83:1617–1642. doi: 10.1007/s11277-015-2467-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Chih-Lin I., Han S., Xu Z., Sun Q., Pan Z. 5G: Rethink mobile communications for 2020+ Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016;374:20140432. doi: 10.1098/rsta.2014.0432. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. International Agency for Research on Cancer . Non-Ionizing Radiation, Part. 2: Radiofrequency Electromagnetic Fields. Volume 102. International Agency for Research on Cancer; Lyon, France: 2013. pp. 1–460. [ Google Scholar ]
- 4. Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) Opinion on: Potential health effects of exposure to electromagnetic fields. Bioelectromagnetics. 2015;36:480–484. doi: 10.1002/bem.21930. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. SSM’s Scientific Council on Electromagnetic Fields . Recent Research on EMF and Health Risk: Eleventh report from SSM’S Scientific Council on Electromagnetic Fields. Swedish Radiation Safety Authority; Stockholm, Sweden: 2016. [ Google Scholar ]
- 6. Marques M.M., Berrington de Gonzalez A., Beland F.A., Browne P., Demers P.A., Lachenmeier D.W., Bahadori T., Barupal D.K., Belpoggi F., Comba P., et al. Advisory Group recommendations on priorities for the IARC Monographs. Lancet Oncol. 2019;20:763–764. doi: 10.1016/S1470-2045(19)30246-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. WHO. [(accessed on 8 August 2019)]; Available online: https://www.who.int/peh-emf/publications/facts/fs226/en/
- 8. Mattsson M.O., Zeni O., Simkó M. Is there a Biological Basis for Therapeutic Applications of Millimetre Waves and THz Waves? J. Infrared Millim. Terahertz Waves. 2018;39:863–878. doi: 10.1007/s10762-018-0483-5. [ DOI ] [ Google Scholar ]
- 9. Ziskin M.C. Millimeter waves: Acoustic and electromagnetic. Bioelectromagnetics. 2013;34:3–14. doi: 10.1002/bem.21750. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. International Commission on Non-Ionizing Radiation Protection (ICNIRP) Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (Up to 300 GHz) Health Phys. 1998;74:494–522. [ PubMed ] [ Google Scholar ]
- 11. IEEE Standards Coordinating Committee . IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3kHz to 300 GHz. IEEE; Piscataway, NJ, USA: 2006. pp. 1–1991. Technical report No. C95.1-2005. [ Google Scholar ]
- 12. IEEE. [(accessed on 8 August 2019)]; Available online: https://spectrum.ieee.org/video/ telecom/wireless/ everything-you-need-to-know-about-5g .
- 13. Zeni O., Scarfì M.R. Microwave Materials Characterization. InTech; Rijeka, Croatia: 2012. Experimental requirements for in vitro studies aimed to evaluate the biological effects of radiofrequency radiation; pp. 121–138. [ Google Scholar ]
- 14. Simkó M., Remondini D., Zeni O., Scarfi M.R. Quality Matters: Systematic Analysis of Endpoints Related to ‘Cellular Life’ in Vitro Data of Radiofrequency Electromagnetic Field Exposure. Int. J. Environ. Res. Public Health. 2016;13:701. doi: 10.3390/ijerph13070701. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. SCHEER–Scientific Committee on Health Environmental and Emerging Risks . Memorandum on Weight of Evidence and Uncertainties. European Comission; Brussels, Belgium: 2018. [ Google Scholar ]
- 16. Alekseev S.I., Gordiienko O.V., Radzievsky A.A., Ziskin M.C. Millimeter wave effects on electrical responses of the sural nerve in vivo. Bioelectromagnetics. 2010;31:180–190. doi: 10.1002/bem.20547. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Alekseev S.I., Radzievsky A.A., Szabo I., Ziskin M.C. Local heating of human skin by millimeter waves: Effect of blood flow. Bioelectromagnetics. 2005;26:489–501. doi: 10.1002/bem.20118. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Bush L.G., Hill D.W., Riazi A., Stensaas L.J., Partlow L.M., Gandhi O.P. Effects of millimeter-wave radiation on monolayer cell cultures. III. A search for frequency-specific athermal biological effects on protein synthesis. Bioelectromagnetics. 1981;2:151–159. doi: 10.1002/bem.2250020206. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Chatterjee I., Yoon J., Wiese R. Millimeter wave bioeffects at 94 GHz on skeletal muscle contraction; Proceedings of the 2013 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems; Austin, TX, USA. 20–23 January 2013; pp. 67–69. [ Google Scholar ]
- 20. Chen Q., Zeng Q.L., Lu D.Q., Chiang H. Millimeter Wave Exposure Reverses TPA Suppression of Gap Junction Intercellular Communication in HaCaT Human Keratinocytes. Bioelectromagnetics. 2004;25:1–4. doi: 10.1002/bem.10140. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. D’Agostino S., Della Monica C., Palizzi E., Di Pietrantonio F., Benetti M., Cannatà D., Cavagnaro M., Sardari D., Stano P., Ramundo-Orlando A. Extremely High Frequency Electromagnetic Fields Facilitate Electrical Signal Propagation by Increasing Transmembrane Potassium Efflux in an Artificial Axon Model. Sci. Rep. 2018;8:9299. doi: 10.1038/s41598-018-27630-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Deghoyan A., Heqimyan A., Nikoghosyan A., Dadasyan E., Ayrapetyan S. Cell bathing medium as a target for non thermal effect of millimeter waves. Electromagn. Biol. Med. 2012;31:132–142. doi: 10.3109/15368378.2011.624659. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Egot-Lemaire S.J.-P., Ziskin M.C. Dielectric properties of human skin at an acupuncture point in the 50–75 GHz frequency range: A pilot study. Bioelectromagnetics. 2011;32:360–366. doi: 10.1002/bem.20650. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Franchini V., Franchini V., Regalbuto E., De Amicis A., De Sanctis S., Di Cristofaro S., Coluzzi E., Marinaccio J., Sgura A., Ceccuzzi S., et al. Genotoxic Effects in Human Fibroblasts Exposed to Microwave Radiation. Health Phys. 2018;115:126–139. doi: 10.1097/HP.0000000000000871. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Frei M.R., Ryan K.L., Berger R.E., Jauchem J.R. Sustained 35-GHz radiofrequency irradiation induces circulatory failure. Shock. 1995;4:289–293. doi: 10.1097/00024382-199510000-00010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Gapeyev A.B., Kulagina T.P., Aripovsky A.V., Chemeris N.K. The role of fatty acids in anti-inflammatory effects of low-intensity extremely high-frequency electromagnetic radiation. Bioelectromagnetics. 2011;32:388–395. doi: 10.1002/bem.20645. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Gapeyev A.B., Gapeyev A.B., Mikhailik E.N., Mikhailik E.N., Chemeris N.K. Anti-inflammatory effects of low-intensity extremely high-frequency electromagnetic radiation: Frequency and power dependence. Bioelectromagnetics. 2008;29:197–206. doi: 10.1002/bem.20381. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Alekseev S.I., Ziskin M.C. Effects of millimeter waves on ionic currents of Lymnaea neurons. Bioelectromagnetics. 1999;20:24–33. doi: 10.1002/(SICI)1521-186X(1999)20:1<24::AID-BEM4>3.0.CO;2-V. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Gapeyev A.B., Safronova V.G., Chemeris N.K., Fesenko E.E. Inhibition of the production of reactive oxygen species in mouse peritoneal neutrophils by millimeter wave radiation in the near and far field zones of the radiator. Bioelectrochem. Bioenerg. 1997;43:217–220. doi: 10.1016/S0302-4598(96)05155-0. [ DOI ] [ Google Scholar ]
- 30. Geletyuk V., Kazachenko V. Dual effects of microwaves on single Ca 2+-activated K+ channels in cultured kidney cells Vero. FEBS Lett. 1995;359:85–88. doi: 10.1016/0014-5793(95)00002-Q. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Grundier W., Keilmann F. Nonthermal Effects of Millimeter Microwaves on Yeast Growth. Z. Naturforsch. C. 1978;33:15–22. doi: 10.1515/znc-1978-1-205. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Haas A.J., Le Page Y., Zhadobov M., Boriskin A., Sauleau R., Le Dréan Y. Impact of 60-GHz millimeter waves on stress and pain-related protein expression in differentiating neuron-like cells. Bioelectromagnetics. 2016;37:444–454. doi: 10.1002/bem.21995. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Haas A.J., Le Page Y., Zhadobov M., Sauleau R., Le Dréan Y., Saligaut C. Effect of acute millimeter wave exposure on dopamine metabolism of NGF-treated PC12 cells. J. Radiat. Res. 2017;58:439–445. doi: 10.1093/jrr/rrx004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Haas A.J., Le Page Y., Zhadobov M., Sauleau R., Le Dréan Y. Effects of 60-GHz millimeter waves on neurite outgrowth in PC12 cells using high-content screening. Neurosci. Lett. 2016;618:58–65. doi: 10.1016/j.neulet.2016.02.038. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Habauzit D., Le Quément C., Zhadobov M., Martin C., Aubry M., Sauleau R., Le Dréan Y. Transcriptome analysis reveals the contribution of thermal and the specific effects in cellular response to millimeter wave exposure. PLoS ONE. 2014;9:e109435. doi: 10.1371/journal.pone.0109435. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Ivanov V.B., Subbotina T.I., Khadartsev A.A., Yashin M.A., Yashin A.A. Exposure to low-intensive superhigh frequency electromagnetic field as a factor of carcinogenesis in experimental animals. Bull. Exp. Biol. Med. 2005;139:241–244. doi: 10.1007/s10517-005-0259-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Jauchem J.R., Ryan K.L., Walters T.J. Pathophysiological alterations induced by sustained 35-GHz radio-frequency energy heating. J. Basic Clin. Physiol. Pharmacol. 2016;27:79–89. doi: 10.1515/jbcpp-2015-0011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Kojima M., Hanazawa M., Yamashiro Y., Sasaki H., Watanabe S., Taki M., Suzuki Y., Hirata A., Kamimura Y., Sasaki K. Acute ocular injuries caused by 60-GHZ millimeter-wave exposure. Heal. Phys. 2009;97:212–218. doi: 10.1097/HP.0b013e3181abaa57. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Alekseev S.I., Ziskin M.C., Kochetkova N.V., Bolshakov M.A. Millimeter waves thermally alter the firing rate of the Lymnaea pacemaker neuron. Bioelectromagnetics. 1997;18:89–98. doi: 10.1002/(SICI)1521-186X(1997)18:2<89::AID-BEM1>3.0.CO;2-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Kojima M., Kojima M., Suzuki Y., Sasaki K., Taki M., Wake K., Watanabe S., Mizuno M., Tasaki T., Sasaki H. Ocular Effects of Exposure to 40, 75, and 95 GHz Millimeter Waves. J. Infrared Millim. Terahertz Waves. 2018;39:912–925. doi: 10.1007/s10762-018-0497-z. [ DOI ] [ Google Scholar ]
- 41. Korenstein-Ilan A., Barbul A., Hasin P., Eliran A., Gover A., Korenstein R. Terahertz Radiation Increases Genomic Instability in Human Lymphocytes. Radiat. Res. 2008;170:224–234. doi: 10.1667/RR0944.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Koschnitzke C., Kremer F., Santo L., Quick P., Poglitsch A. A Non-Thermal Effect of Millimeter Wave Radiation on the Puffing of Giant Chromosomes. Zeitschrift für Naturforsch C. 1983;38:883–886. doi: 10.1515/znc-1983-9-1038. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Koyama S., Narita E., Shimizu Y., Suzuki Y., Shiina T., Taki M., Shinohara M., Miyakoshi J. Effects of long-term exposure to 60 GHz millimeter-wavelength radiation on the genotoxicity and heat shock protein (HSP) expression of cells derived from human eye. Int. J. Environ. Res. Public Health. 2016;13:802. doi: 10.3390/ijerph13080802. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Kues H.A., Anna S.A.D., Osiander R., Green W.R., Monahan J.C. Absence of Ocular Effects After Either Single or Repeated Exposure to 10 mW/cm2 from a 60 GHz CW Source. Bioelectromagnetics. 1999;473:463–473. doi: 10.1002/(SICI)1521-186X(199912)20:8<463::AID-BEM1>3.0.CO;2-T. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Le Quément C., Nicolas Nicolaz C., Zhadobov M., Desmots F., Sauleau R., Aubry M., Michel D., Le Dréan Y. Whole-genome expression analysis in primary human keratinocyte cell cultures exposed to 60 GHz radiation. Bioelectromagnetics. 2012;33:147–158. doi: 10.1002/bem.20693. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. LeQuément C., Nicolaz C.N., Habauzit D., Zhadobov M., Sauleau R., Le Dréan Y. Impact of 60-GHz millimeter waves and corresponding heat effect on endoplasmic reticulum stress sensor gene expression. Bioelectromagnetics. 2014;35:444–451. doi: 10.1002/bem.21864. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Li X., Ye H., Yu F., Cai L., Li H., Chen J., Wu M., Chen W., Lin R., Li Z., et al. Millimeter wave treatment promotes chondrocyte proliferation via G 1/S cell cycle transition. Int. J. Mol. Med. 2012;29:823–831. doi: 10.3892/ijmm.2012.919. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Logani M.K., Agelan A., Ziskin M.C. Effect of millimeter wave radiation on catalase activity. Electromagn. Biol. Med. 2002;21:303–308. doi: 10.1081/JBC-120016009. [ DOI ] [ Google Scholar ]
- 49. Logani M.K., Anga A., Szabo I., Agelan A., Irizarry A.R., Ziskin M.C. Effect of millimeter waves on cyclophosphamide induced suppression of the immune system. Bioelectromagnetics. 2002;23:614–621. doi: 10.1002/bem.10058. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Alekseev S.I., Ziskin M.C. Millimeter microwave effect on ion transport across lipid bilayer membranes. Bioelectromagnetics. 1995;16:124–131. doi: 10.1002/bem.2250160209. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Makar V., Logani M., Szabo I., Zlskin M. Effect of Millimeter Waves on Cyclophosphamide Induced Suppression of T Cell Functions. Bioelectromagnetics. 2003;24:356–365. doi: 10.1002/bem.10106. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Makar V.R., Logani M.K., Bhanushali A., Alekseev S.I., Ziskin M.C. Effect of cyclophosphamide and 61.22 GHz millimeter waves on T-cell, B-cell, and macrophage functions. Bioelectromagnetics. 2006;27:458–466. doi: 10.1002/bem.20230. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Makar V.R., Logani M.K., Bhanushali A., Kataoka M., Ziskin M.C. Effect of millimeter waves on natural killer cell activation. Bioelectromagnetics. 2005;26:10–19. doi: 10.1002/bem.20046. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Mason P.A., Walters T.J., DiGiovanni J., Beason C.W., Jauchem J.R., Dick E.J., Jr., Mahajan K., Dusch S.J., Shields B.A., Merritt J.H., et al. Lack of effect of 94 GHz radio frequency radiation exposure in an animal model of skin carcinogenesis in the radio frequency radiation (RFR) band is mutagenic, as either a promoter or co-promoter in some animal models of carcinogenesis. Recent develop. Carcinogenesis. 2001;22:1701–1708. doi: 10.1093/carcin/22.10.1701. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Millenbaugh N.J., Kiel J.L., Ryan K.L., Blystone R.V., Kalns J.E., Brott B.J., Cerna C.Z., Lawrence W.S., Soza L.L., Mason P.A. Comparison of blood pressure and thermal responses in rats exposed to millimeter wave energy or environmental heat. Shock. 2006;25:625–632. doi: 10.1097/01.shk.0000209550.11087.fd. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Millenbaugh N.J., Roth C., Sypniewska R., Chan V., Eggers J.S., Kiel J.L., Blystone R.V., Mason P.A. Gene expression changes in the skin of rats induced by prolonged 35 GHz millimeter-wave exposure. Radiat. Res. 2008;169:288–300. doi: 10.1667/RR1121.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Narinyan L., Ayrapetyan S. Cyclic AMP-dependent signaling system is a primary metabolic target for non-thermal effect of microwaves on heart muscle hydration. Electromagn. Biol. Med. 2017;36:182–191. doi: 10.1080/15368378.2016.1241803. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Nguyen T.H., Pham V.T., Nguyen S.H., Baulin V., Croft R.J., Phillips B., Crawford R.J., Ivanova E.P. The bioeffects resulting from prokaryotic cells and yeast being exposed to an 18 GHz electromagnetic field. PLoS ONE. 2016;11:e0158135. doi: 10.1371/journal.pone.0158135. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Nicolaz C.N., Zhadobov M., Desmots F., Ansart A., Sauleau R., Thouroude D., Michel D., Le Drean Y. Study of narrow band millimeter-wave potential interactions with endoplasmic reticulum stress sensor genes. Bioelectromagnetics. 2009;30:365–373. doi: 10.1002/bem.20481. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Nicolas Nicolaz C., Zhadobov M., Desmots F., Sauleau R., Thouroude D., Michel D., Le Drean Y. Absence of direct effect of low-power millimeter-wave radiation at 60.4 GHz on endoplasmic reticulum stress. Cell Biol. Toxicol. 2009;25:471–478. doi: 10.1007/s10565-008-9101-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Bellossi A., Dubost G., Moulinoux J.P., Himdi M., Ruelloux M., Rocher C. Biological Effects of Millimeter-Wave Irradiation on Mice—Preliminary Results. IEEE Trans. Microw. Theory Tech. 2000;48:2104–2110. [ Google Scholar ]
- 62. Pakhomov A.G., Prol H.K., Mathur S.P., Akyel Y., Campbell C.B.G.B.G. Role of field intensity in the biological effectiveness of millimeter waves at a resonance frequency. Bioelectrochem. Bioenerg. 1997;43:27–33. doi: 10.1016/S0302-4598(97)00022-6. [ DOI ] [ Google Scholar ]
- 63. Pakhomov A.G., Prol H.K., Mathur S.P., Akyel Y., Campbell C.B. Search for frequency-specific effects of millimeter-wave radiation on isolated nerve function. Bioelectromagnetics. 1997;18:324–334. doi: 10.1002/(SICI)1521-186X(1997)18:4<324::AID-BEM5>3.0.CO;2-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Partlow L.M., Bush L.G., Stensaas L.J., Hill D.W., Riazi A., Gandhi O.P. Effects of millimeter-wave radiation on monolayer cell cultures. I. Design and validation of a novel exposure system. Bioelectromagnetics. 1981;2:123–140. doi: 10.1002/bem.2250020204. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Partyla T., Hacker H., Edinger H., Leutzow B., Lange J., Usichenko T. Remote Effects of Electromagnetic Millimeter Waves on Experimentally Induced Cold Pain: A Double-Blinded Crossover Investigation in Healthy Volunteers. Anesth. Analg. 2017;124:980–985. doi: 10.1213/ANE.0000000000001657. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Pikov V., Arakaki X., Harrington M., Fraser S.E., Siegel P.H. Modulation of neuronal activity and plasma membrane properties with low-power millimeter waves in organotypic cortical slices. J. Neural Eng. 2010;7:045003. doi: 10.1088/1741-2560/7/4/045003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Radzievsky A., Gordiienko O., Cowan A., Alekseev S., Ziskin M. Millimeter-Wave-Induced Hypoalgesia in Mice: Dependence on Type of Experimental Pain. IEEE Trans. Plasma Sci. 2004;32:1634–1643. doi: 10.1109/TPS.2004.830972. [ DOI ] [ Google Scholar ]
- 68. Radzievsky A.A., Cowan A., Byrd C., Radzievsky A.A., Ziskin M.C. Single millimeter wave treatment does not impair gastrointestinal transit in mice. Life Sci. 2002;71:1763–1770. doi: 10.1016/S0024-3205(02)01944-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Radzievsky A.A., Gordiienko O.V., Alekseev S., Szabo I., Cowan A., Ziskin M.C. Electromagnetic millimeter wave induced hypoalgesia: Frequency dependence and involvement of endogenous opioids. Bioelectromagnetics. 2008;29:284–295. doi: 10.1002/bem.20389. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Radzievsky A., Gordiienko O., Szabo I., Alekseev S.I., Ziskin M.C. Millimeter wave-induced suppression of B16 F10 melanoma growth in mice: Involvement of endogenous opioids. Bioelectromagnetics. 2004;25:466–473. doi: 10.1002/bem.20018. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Radzievsky A.A., Rojavin M.A., Cowan A., Alekseev S.I., Radzievsky A.A., Ziskin M.C. Peripheral neural system involvement in hypoalgesic effect of electromagnetic millimeter waves. Life Sci. 2001;62:1143–1151. doi: 10.1016/S0024-3205(00)01016-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Beneduci A., Chidichimo G., De Rose R., Filippelli L., Straface S.V., Venuta S. Frequency and irradiation time-dependant antiproliferative effect of low-power millimeter waves on RPMI 7932 human melanoma cell line. Anticancer Res. 2005;25:1023–1028. [ PubMed ] [ Google Scholar ]
- 73. Radzievsky A.A., Rojavin M.A., Cowan A., Alekseev S.I., Ziskin M.C. Hypoalgesic effect of millimeter waves in mice: Dependence on the site of exposure. Life Sci. 2000;66:2101–2111. doi: 10.1016/S0024-3205(00)00536-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Radzievsky A.A., Rojavin M.A., Cowan A., Ziskin M.C. Suppression of pain sensation caused by millimeter waves: A double-blinded, cross-over, prospective human volunteer study. Anesth. Analg. 1999;88:836–840. doi: 10.1213/00000539-199904000-00029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Rojavin M.A., Cowan A., Radzievsky A.A., Ziskin M.C. Antipruritic effect of millimeter waves in mice: Evidence for opioid involvement. Life Sci. 1998;63:251–257. doi: 10.1016/S0024-3205(98)00436-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Rojavin M.A., Radzievsky A.A., Cowan A., Ziskin M.C. Pain relief caused by millimeter waves in mice: Results of cold water tail flick tests. Int. J. Radiat. Biol. 2000;76:575–579. doi: 10.1080/095530000138592. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Romanenko S., Siegel P.H., Wagenaar D.A., Pikov V. Effects of millimeter wave irradiation and equivalent thermal heating on the activity of individual neurons in the leech ganglion. J. Neurophysiol. 2014;112:2423–2431. doi: 10.1152/jn.00357.2014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Ryan K.L., Frei M.R., Berger R.E., Jauchem J.R. Does nitric oxide mediate circulatory failure induced by 35-GHz microwave heating? Shock. 1996;6:71–76. doi: 10.1097/00024382-199607000-00015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Ryan K.L., Frei M.R., Jauchem J.R. Circulatory failure induced by 35 GHz microwave heating: Effects of chronic nitric oxide synthesis inhibition. Shock. 1997;7:70–76. doi: 10.1097/00024382-199701000-00010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Safronova V.G., Gabdoulkhakova A.G., Santalov B.F. Immunomodulating Action of Low Intensity Millimeter Waves on Primed Neutrophils. Bioelectromagnetics. 2002;23:599–606. doi: 10.1002/bem.10056. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Samoilov V.O., Shadrin E.B., Filippova E.B., Katsnelson Y., Backhoff H., Eventov M. The effect of transcranial electromagnetic brain stimulation on the acquisition of the conditioned response in rats. Biophysics. 2015;60:303–308. doi: 10.1134/S0006350915020165. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Samsonov A., Popov S.V. The effect of a 94 GHz electromagnetic field on neuronal microtubules. Bioelectromagnetics. 2013;34:133–144. doi: 10.1002/bem.21760. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Beneduci A., Chidichimo G., Tripepi S., Perrotta E. Transmission electron microscopy study of the effects produced by wide-band low-power millimeter waves on MCF-7 human breast cancer cells in culture. Anticancer Res. 2005;25:1009–1013. [ PubMed ] [ Google Scholar ]
- 84. Shanin S.N., Rybakina E.G., Novikova N.N., Kozinets I.A., Rogers V.J., Korneva E.A. Natural killer cell cytotoxic activity and c-Fos protein synthesis in rat hypothalamic cells after painful electric stimulation of the hind limbs and EHF irradiation of the skin. Med. Sci Monit. 2005;11:BR309–BR315. [ PubMed ] [ Google Scholar ]
- 85. Shapiro M.G., Priest M.F., Siegel P.H., Bezanilla F. Thermal Mechanisms of Millimeter Wave Stimulation of Excitable Cells. Biophys. J. 2013;104:2622–2628. doi: 10.1016/j.bpj.2013.05.014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Shckorbatov Y.G., Grigoryeva N.N., Shakhbazov V.G., Grabina V.A., Bogoslavsky A.M. Microwave irradiation influences on the state of human cell nuclei. Bioelectromagnetics. 1998;19:414–419. doi: 10.1002/(SICI)1521-186X(1998)19:7<414::AID-BEM2>3.0.CO;2-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Sivachenko I.B., Medvedev D.S., Molodtsova I.D., Panteleev S.S., Sokolov A.Y., Lyubashina O.A. Effects of Millimeter-Wave Electromagnetic Radiation on the Experimental Model of Migraine. Bull. Exp. Biol. Med. 2016;160:425–428. doi: 10.1007/s10517-016-3187-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Soubere Mahamoud Y., Aite M., Martin C., Zhadobov M., Sauleau R., Le Dréan Y., Habauzit D. Additive Effects of Millimeter Waves and 2-Deoxyglucose Co-Exposure on the Human Keratinocyte Transcriptome. PLoS ONE. 2016;11:e0160810. doi: 10.1371/journal.pone.0160810. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Stensaas L.J., Partlow L.M., Bush L.G., Iversen P.L., Hill D.W., Hagmann M.J., Gandhi O.P. Effects of millimeter-wave radiation on monolayer cell cultures. II. Scanning and transmission electron microscopy. Bioelectromagnetics. 1981;2:141–150. doi: 10.1002/bem.2250020205. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Sun S., Titushkin I., Varner J., Cho M. Millimeter Wave-induced Modulation of Calcium Dynamics in an Engineered Skin Co-culture Model: Role of Secreted ATP on Calcium Spiking. J. Radiat. Res. 2012;53:159–167. doi: 10.1269/jrr.11037. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Sypniewska R.K., Millenbaugh N.J., Kiel J.L., Blystone R.V., Ringham H.N., Mason P.A., Witzmann F.A. Protein changes in macrophages induced by plasma from rats exposed to 35 GHz millimeter waves. Bioelectromagnetics. 2010;31:656–663. doi: 10.1002/bem.20598. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Szabo I., Kappelmayer J., Alekseev S.I., Ziskin M.C. Millimeter wave induced reversible externalization of phosphatidylserine molecules in cells exposed in vitro. Bioelectromagnetics. 2006;27:233–244. doi: 10.1002/bem.20202. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Szabo I., Manning M.R., Radzlevsky A.A., Wetzel M.A., Rogers T.J., Ziskin M.C. Low Power Millimeter Wave Irradiation Exerts No Harmful Effect on Human Keratinocytes In Vitro. Bioelectromagnetics. 2003;24:165–173. doi: 10.1002/bem.10077. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Beneduci A., Chidichimo G., Tripepi S., Perrotta E., Cufone F. Antiproliferative effect of millimeter radiation on human erythromyeloid leukemia cell line K562 in culture: Ultrastructural and metabolic-induced changes. Bioelectrochemistry. 2007;70:214–220. doi: 10.1016/j.bioelechem.2006.07.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Szabo I., Rojavin M.A., Rogers T.J., Ziskin M.C. Reactions of keratinocytes to in vitro millimeter wave exposure. Bioelectromagnetics. 2001;22:358–364. doi: 10.1002/bem.62. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Titushkin I.A., Rao V.S., Pickard W.F., Moros E.G., Shafirstein G., Cho M.R. Altered Calcium Dynamics Mediates P19-Derived Neuron-Like Cell Responses to Millimeter-Wave Radiation. Radiat. Res. 2009;172:725–763. doi: 10.1667/RR1760.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Tong Y., Yang Z., Yang D., Chu H., Qu M., Liu G., Wu Y., Liu S. Millimeter-wave exposure promotes the differentiation of bone marrow stromal cells into cells with a neural phenotype. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2009;29:409–412. doi: 10.1007/s11596-009-0403-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Logani M.K., Bhanushali A., Ziskin M.C., Prihoda T.J. Micronuclei in peripheral blood and bone marrow cells of mice exposed to 42 GHz electromagnetic millimeter waves. Radiat. Res. 2004;161:341–345. doi: 10.1667/RR3121. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Vlasova I.I., Mikhalchik E.V., Gusev A.A., Balabushevich N.G., Gusev S.A., Kazarinov K.D. Extremely high-frequency electromagnetic radiation enhances neutrophil response to particulate agonists. Bioelectromagnetics. 2018;39:144–155. doi: 10.1002/bem.22103. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Volkova N.A., Pavlovich E.V., Gapon A.A., Nikolov O.T. Effects of millimeter-wave electromagnetic exposure on the morphology and function of human cryopreserved spermatozoa. Bull. Exp. Biol. Med. 2014;157:574–576. doi: 10.1007/s10517-014-2618-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Wu G., Sferra T., Chen X., Chen Y., Wu M., Xu H., Peng J., Liu X. Millimeter wave treatment inhibits the mitochondrion-dependent apoptosis pathway in chondrocytes. Mol. Med. Rep. 2011;4:1001–1006. doi: 10.3892/mmr.2011.522. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Wu G.W., Liu X.X., Wu M.X., Zhao J.Y., Chen W.L., Lin R.H., Lin J.M. Experimental study of millimeter wave-induced differentiation of bone marrow mesenchymal stem cells into chondrocytes. Int. J. Mol. Med. 2009;23:461–467. doi: 10.3892/ijmm_00000152. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Xia L., Luo Q.-L., Lin H.-D., Zhang J.-L., Guo H., He C.-Q. The effect of different treatment time of millimeter wave on chondrocyte apoptosis, caspase-3, caspase-8, and MMP-13 expression in rabbit surgically induced model of knee osteoarthritis. Rheumatol. Int. 2012;32:2847–2856. doi: 10.1007/s00296-011-2080-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Xie T., Pei J., Cui Y., Zhang J., Qi H., Chen S., Qiao D. EEG changes as heat stress reactions in rats irradiated by high intensity 35 GHZ millimeter waves. Health Phys. 2011;100:632–640. doi: 10.1097/HP.0b013e3182027d10. [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Beneduci A. Evaluation of the Potential In Vitro Antiproliferative Effects of Millimeter Waves at Some Therapeutic Frequencies on RPMI 7932 Human Skin Malignant Melanoma Cells. Cell Biochem. Biophys. 2009;55:25–32. doi: 10.1007/s12013-009-9053-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Yaekashiwa N., Otsuki S., Hayashi S., Kawase K. Investigation of the non-thermal effects of exposing cells to 70-300 GHz irradiation using a widely tunable source. J. Radiat. Res. 2018;59:116–121. doi: 10.1093/jrr/rrx075. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 107. Yu G., Coln E.A., Schoenbach K.H., Gellerman M., Fox P., Rec L., Beebe S.J., Liu S. A study on biological effects of low-intensity millimeter waves. IEEE Trans. Plasma Sci. 2002;30:1489–1496. [ Google Scholar ]
- 108. Zhadobov M., Desmots F., Thouroude D., Michel D., Drean Y. Evaluation of the potential biological effects of the 60-GHz millimeter waves upon human cells. IEEE Trans. Antennas Propag. 2009;57:2949–2956. doi: 10.1109/TAP.2009.2029308. [ DOI ] [ Google Scholar ]
- 109. Zhadobov M., Sauleau R., Le Coq L., Debure L., Thouroude D., Michel D., Le Dréan Y. Low-power millimeter wave radiations do not alter stress-sensitive gene expression of chaperone proteins. Bioelectromagnetics. 2007;28:188–196. doi: 10.1002/bem.20285. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Aminzadeh R., Thielens A., Bamba A., Kone L., Gaillot D.P., Lienard M., Martens L., Joseph W. On-body calibration and measurements using personal radiofrequency exposimeters in indoor diffuse and specular environments. Bioelectromagnetics. 2016;37:298–309. doi: 10.1002/bem.21975. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Colombi D., Thors B., Tornevik C., Balzano Q. RF Energy Absorption by Biological Tissues in Close Proximity to Millimeter-Wave 5G Wireless Equipment. IEEE Access. 2018;6:4974–4981. doi: 10.1109/ACCESS.2018.2790038. [ DOI ] [ Google Scholar ]
- 112. Neufeld E., Carrasco E., Murbach M., Balzano Q., Christ A., Kuster N. Theoretical and numerical assessment of maximally allowable power-density averaging area for conservative electromagnetic exposure assessment above 6 GHz. Bioelectromagnetics. 2018;39:617–630. doi: 10.1002/bem.22147. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Foster K.R., Ziskin M.C., Balzano Q. Thermal modeling for the next generation of radiofrequency exposure limits: Commentary. Health Phys. 2017;113:41–53. doi: 10.1097/HP.0000000000000671. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Foster K.R., Ziskin M.C., Balzano Q. Thermal Response of Human Skin to Microwave Energy: A Critical Review. Health Phys. 2016;111:528–541. doi: 10.1097/HP.0000000000000571. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Zhadobov M., Alekseev S.I., Sauleau R., Le Page Y., Le Drean Y., Fesenko E.E. Microscale temperature and SAR measurements in cell monolayer models exposed to millimeter waves. Bioelectromagnetics. 2017;38:11–21. doi: 10.1002/bem.21999. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Alekseev S.I., Gordiienko O.V., Ziskin M.C. Reflection and penetration depth of millimeter waves in murine skin. Bioelectromagnetics. 2008;29:340–344. doi: 10.1002/bem.20401. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Alekseev S.I., Radzievsky A.A., Logani M.K., Ziskin M.C. Millimeter wave dosimetry of human skin. Bioelectromagnetics. 2008;29:65–70. doi: 10.1002/bem.20363. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Alekseev S.I., Ziskin M.C., Fesenko E.E. Frequency dependence of heating of human skin exposed to millimeter waves. Biophysics. 2012;57:90–93. doi: 10.1134/S0006350912010046. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Laakso I., Morimoto R., Heinonen J., Jokela K., Hirata A. Human exposure to pulsed fields in the frequency range from 6 to 100 GHz. Phys. Med. Biol. 2017;62:6980–6992. doi: 10.1088/1361-6560/aa81fe. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Feldman Y., Puzenko A., Ben Ishai P., Caduff A., Agranat A.J. Human skin as arrays of helical antennas in the millimeter and submillimeter wave range; Proceedings of the 2008 33rd International Conference on Infrared, Millimeter and Terahertz Waves; Pasadena, CA, USA. 27 March 2008; pp. 1–2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Shafirstein G., Moros E.G. Modelling millimetre wave propagation and absorption in a high resolution skin model: The effect of sweat glands. Phys. Med. Biol. 2011;56:1329. doi: 10.1088/0031-9155/56/5/007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 122. Alekseev S.I., Ziskin M.C. Enhanced absorption of millimeter wave energy in murine subcutaneous blood vessels. Bioelectromagnetics. 2011;32:423–433. doi: 10.1002/bem.20658. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 123. Sasaki K., Wake K., Watanabe S. Measurement of the dielectric properties of the epidermis and dermis at frequencies from 0.5 GHz to 110 GHz. Phys. Med. Biol. 2014;59:4739–4747. doi: 10.1088/0031-9155/59/16/4739. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Federal Communications Commission (FCC) [(accessed on 8 August 2019)]; Available online: https://www.fcc.gov /general/ radio-frequency-safety-0 .
- 125. Foster K.R., Morrissey J.J. Thermal aspects of exposure to radiofrequency energy: Report of a workshop. Int. J. Hyperth. 2011;27:307–319. doi: 10.3109/02656736.2010.545965. [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (1.2 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 16 March 2021

5G mobile networks and health—a state-of-the-science review of the research into low-level RF fields above 6 GHz
- Ken Karipidis ORCID: orcid.org/0000-0001-7538-7447 1 ,
- Rohan Mate 1 ,
- David Urban 1 ,
- Rick Tinker 1 &
- Andrew Wood 2
Journal of Exposure Science & Environmental Epidemiology volume 31 , pages 585–605 ( 2021 ) Cite this article
187k Accesses
78 Citations
386 Altmetric
Metrics details
The increased use of radiofrequency (RF) fields above 6 GHz, particularly for the 5 G mobile phone network, has given rise to public concern about any possible adverse effects to human health. Public exposure to RF fields from 5 G and other sources is below the human exposure limits specified by the International Commission on Non-Ionizing Radiation Protection (ICNIRP). This state-of-the science review examined the research into the biological and health effects of RF fields above 6 GHz at exposure levels below the ICNIRP occupational limits. The review included 107 experimental studies that investigated various bioeffects including genotoxicity, cell proliferation, gene expression, cell signalling, membrane function and other effects. Reported bioeffects were generally not independently replicated and the majority of the studies employed low quality methods of exposure assessment and control. Effects due to heating from high RF energy deposition cannot be excluded from many of the results. The review also included 31 epidemiological studies that investigated exposure to radar, which uses RF fields above 6 GHz similar to 5 G. The epidemiological studies showed little evidence of health effects including cancer at different sites, effects on reproduction and other diseases. This review showed no confirmed evidence that low-level RF fields above 6 GHz such as those used by the 5 G network are hazardous to human health. Future experimental studies should improve the experimental design with particular attention to dosimetry and temperature control. Future epidemiological studies should continue to monitor long-term health effects in the population related to wireless telecommunications.
Similar content being viewed by others
Meta-analysis of in vitro and in vivo studies of the biological effects of low-level millimetre waves
Radio-frequency electromagnetic field exposure and contribution of sources in the general population: an organ-specific integrative exposure assessment
A study of the long term changes in the electromagnetic environment using data from continuous monitoring sensors in Greece
Introduction.
There are continually emerging technologies that use radiofrequency (RF) electromagnetic fields particularly in telecommunications. Most telecommunication sources currently operate at frequencies below 6 GHz, including radio and TV broadcasting and wireless sources such as local area networks and mobile telephony. With the increasing demand for higher data rates, better quality of service and lower latency to users, future wireless telecommunication sources are planned to operate at frequencies above 6 GHz and into the ‘millimetre wave’ range (30–300 GHz) [ 1 ]. Frequencies above 6 GHz have been in use for many years in various applications such as radar, microwave links, airport security screening and in medicine for therapeutic applications. However, the planned use of millimetre waves by future wireless telecommunications, particularly the 5th generation (5 G) of mobile networks, has given rise to public concern about any possible adverse effects to human health.
The interaction mechanisms of RF fields with the human body have been extensively described and tissue heating is the main effect for RF fields above 100 kHz (e.g. HPA; SCENHIR) [ 2 , 3 ]. RF fields become less penetrating into body tissue with increasing frequency and for frequencies above 6 GHz the depth of penetration is relatively short with surface heating being the predominant effect [ 4 ].
International exposure guidelines for RF fields have been developed on the basis of current scientific knowledge to ensure that RF exposure is not harmful to human health [ 5 , 6 ]. The guidelines developed by the International Commission on Non-Ionizing Radiation Protection (ICNIRP) in particular form the basis for regulations in the majority of countries worldwide [ 7 ]. In the frequency range above 6 GHz and up to 300 GHz the ICNIRP guidelines prevent excessive heating at the surface of the skin and in the eye.
Although not as extensively studied as RF fields at lower frequencies, a number of studies have investigated the effects of RF fields at frequencies above 6 GHz. Previous reviews have reported studies investigating frequencies above 6 GHz that show effects although many of the reported effects occurred at levels greater than the ICNIRP guidelines [ 1 , 8 ]. Given the public concern over the planned roll-out of 5 G using millimetre waves, it is important to determine whether there are any related adverse health consequences at levels encountered in the environment. The aim of this paper is to present a state-of-the-science review of the bioeffects research into RF fields above 6 GHz at low levels of exposure (exposure below the occupational limits of the ICNIRP guidelines). A meta-analysis of in vitro and in vivo studies, providing quantitative effect estimates for each study, is presented separately in a companion paper [ 9 ].
The state-of-the-science review included a comprehensive search of all available literature and examined the extent, range and nature of evidence into the bioeffects of RF fields above 6 GHz, at levels below the ICNIRP occupational limits. The review consisted of biomedical studies on low-level RF electromagnetic fields from 6 GHz to 300 GHz published at any starting date up to December 2019. Studies were initially found by searching the databases PubMed, EMF-Portal, Google Scholar, Embase and Web of Science using the search terms “millimeter wave”, “millimetre wave”, “gigahertz”, “GHz” and “radar”. We further searched major reviews published by health authorities on RF and health [ 2 , 3 , 10 , 11 ]. Finally, we searched the reference list of all the studies included. Studies were only included if the full paper was available in English.
Although over 300 studies were considered, this review was limited to experimental studies (in vitro, in vivo, human) where the stated RF exposure level was at or below the occupational whole-body limits specified by the ICNIRP (2020) guidelines: power density (PD) reference level of 50 W/m 2 or specific absorption rate (SAR) basic restriction of 0.4 W/kg. Since the PD occupational limits for local exposure are more relevant to in vitro studies, and since these limits are higher, we have included those studies with PD up to 100–200 W/m 2 , depending on frequency. The review included studies below the ICNIRP general public limits that are lower than the occupational limits.
The review also included epidemiological studies (cohort, case-control, cross-sectional) investigating exposure to radar but excluded studies where the stated radar frequencies were below 6 GHz. Epidemiological studies on radar were included as they represent occupational exposure below the ICNIRP guidelines. Case reports or case series were excluded. Studies investigating therapeutical outcomes were also excluded unless they reported specific bio-effects.
The state-of-the-science review appraised the quality of the included studies, but unlike a systematic review it did not exclude any studies based on quality. The review also identified gaps in knowledge for future investigation and research. The reporting of results in this paper is narrative with tabular accompaniment showing study characteristics. In this paper, the acronym “MMWs” (or millimetre waves) is used to denote RF fields above 6 GHz.
The review included 107 experimental studies (91 in vitro, 15 in vivo, and 1 human) that investigated various bioeffects, including genotoxicity, cell proliferation, gene expression, cell signalling, membrane function and other effects. The exposure characteristics and biological system investigated in experimental studies for the various bioeffects are shown in Tables 1 – 6 . The results of the meta-analysis of the in vitro and in vivo studies are presented separately in Wood et al. [ 9 ].
Genotoxicity
Studies have examined the effects of exposing whole human or mouse blood samples or lymphocytes and leucocytes to low-level MMWs to determine possible genotoxicity. Some of the genotoxicity studies have looked at the possible effects of MMWs on chromosome aberrations [ 12 , 13 , 14 ]. At exposure levels below the ICNIRP limits, the results have been inconsistent, with either a statistically significant increase [ 14 ] or no significant increase [ 12 , 13 ] in chromosome aberrations.
MMWs do not penetrate past the skin therefore epithelial and skin cells have been a common model of examination for possible genotoxic effects. DNA damage in a number of epithelial and skin cell types and at varied exposure parameters both below and above the ICNIRP limits have been examined using comet assays [ 15 , 16 , 17 , 18 , 19 ]. Despite the varied exposure models and methods used, no statistically significant evidence of DNA damage was identified in these studies. Evidence of genotoxic damage was further assessed in skin cells by the occurrence of micro-nucleation. De Amicis et al. [ 18 ] and Franchini et al. [ 19 ] reported a statistically significant increase in micro-nucleation, however, Hintzsche et al. [ 15 ] and Koyama et al. [ 16 , 17 ] did not find an effect. Two of the studies also examined telomere length and found no statistically significant difference between exposed and unexposed cells [ 15 , 19 ]. Last, a Ukrainian research group examined different skin cell types in three studies and reported an increase in chromosome condensation in the nucleus [ 20 , 21 , 22 ]; these results have not been independently verified. Overall, there was no confirmed evidence of MMWs causing genotoxic damage in epithelial and skin cells.
Three studies from an Indian research group have examined indicators of DNA damage and reactive oxygen species (ROS) production in rats exposed in vivo to MMWs. The studies reported DNA strand breaks based on evidence from comet assays [ 23 , 24 ] and changes in enzymes that control the build-up of ROS [ 24 ]. Kumar et al. also reported an increase in ROS production [ 25 ]. All the studies from this research group had low animal numbers (six animals exposed) and their results have not been independently replicated. An in vitro study that investigated ROS production in yeast cultures reported an increase in free radicals exposed to high-level but not low-level MMWs [ 26 ].
Other studies have looked at the effect of low-level MMWs on DNA in a range of different ways. Two studies reported that MMWs induce colicin synthesis and prophage induction in bacterial cells, both of which are suggested as indicative of DNA damage [ 27 , 28 ]. Another study suggested that DNA exposed to MMWs undergoes polymerase chain reaction synthesis differently than unexposed DNA [ 29 ], although no statistical analysis was presented. Hintzsche et al. reported statistically significant occurrence of spindle disturbance in hybrid cells exposed to MMWs [ 30 ]. Zeni et al. found no evidence of DNA damage or alteration of cell cycle kinetics in blood cells exposed to MMWs [ 31 ]. Last, two studies from a Russian research group examined the protective effects of MMWs where mouse blood leukocytes were pre-exposed to low-level MMWs and then to X-rays [ 32 , 33 ]. The studies reported that there was statistically significant less DNA damage in the leucocytes that were pre-exposed to MMWs than those exposed to X-rays alone. Overall, these studies had no independent replication.
Cell proliferation
A number of studies have examined the effects of low-level MMWs on cell proliferation and they have used a variety of cellular models and methods of investigation. Studies have exposed bacterial cells to low-level MMWs alone or in conjunction with other agents. Two early studies reported changes in the growth rate of E. coli cultures exposed to low-level MMWs; however, both of these studies were preliminary in nature without appropriate dosimetry or statistical analysis [ 34 , 35 ]. Two studies exposed E. coli cultures and one study exposed yeast cell cultures to MMWs alone, and before and after UVC exposure [ 36 , 37 , 38 ]. All three studies reported that MMWs alone had no significant effect on bacterial cell proliferation or survival. Rojavin et al., however, did report that when E. coli bacteria were exposed to MMWs after UVC sterilisation treatment, there was an increase in their survival rate [ 36 ]. The authors suggested this could be due to the MMW activation of bacterial DNA repair mechanisms. Other studies by an Armenian research group reported a reduction in E. coli cell growth when exposed to MMWs [ 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. These studies reported that when E.coli cultures were exposed to MMWs in the presence of antibiotics, there was a greater reduction in the bacterial growth rate and an increase in the time between bacterial cell division compared with antibiotics exposure alone. Two of these studies investigated if these effects could be due to a reduction in the activity of the E. coli ATPase when exposed to MMWs. The studies reported exposure to MMWs in combination with particular antibiotics changed the concentration of H + and K + ions in the E.coli cells, which the authors linked to changes in ATPase activity [ 43 , 44 ]. Overall, the results from studies on cell proliferation of bacterial cells have been inconsistent with different research groups reporting conflicting results.
Studies have also examined how exposure to low-level MMWs could affect cell proliferation in yeast. Two early studies by a German research group reported changes in yeast cell growth [ 46 , 47 ]. However, another two independent studies did not report any changes in the growth rate of exposed yeast [ 48 , 49 ]. Furia et al. [ 48 ] noted that the Grundler and Keilmann studies [ 46 , 47 ] had a number of methodical issues, which may have skewed their results, such as poor exposure control and analysis of results. Another study exposed yeast to MMWs before and after UVC exposure and reported that MMWs did not change the rates of cell survival [ 37 ].
Studies have also examined the possible effect of low-level MMWs on tumour cells with some studies reporting a possible anti-proliferative effect. Chidichimo et al. reported a reduction in the growth of a variety of tumour cells exposed to MMWs; however, the results of the study did not support this conclusion [ 50 ]. An Italian research group published a number of studies investigating proliferation effects on human melanoma cell lines with conflicting results. Two of the studies reported reduced growth rate [ 51 , 52 ] and a third study showed no change in proliferation or in the cell cycle [ 53 ]. Beneduci et al. also reported changes in the morphology of MMW exposed cells; however, the authors did not present quantitative data for these reported changes [ 51 , 52 ]. In another study by the same Italian group, Beneduci et al. reported that exposure to low-level MMWs had a greater than 40% reduction in the number of viable erythromyeloid leukaemia cells compared with controls; however, there was no significant change in the number of dead cells [ 54 ]. More recently, Yaekashiwa et al. reported no statistically significant effect in proliferation or cellular activity in glioblastoma cells exposed to low-level MMWs [ 55 ].
Other studies did not report statistically significant effects on proliferation in chicken embryo cell cultures, rat nerve cells or human skin fibroblasts exposed to low-level MMWs [ 55 , 56 , 57 ].
Gene expression
Some studies have investigated whether low-level MMWs can influence gene expression. Le Queument et al. examined a multitude of genes using microarray analyses and reported transient expression changes in five of them. However, the authors concluded that these results were extremely minor, especially when compared with studies using microarrays to study known pollutants [ 58 ]. Studies by a French research group have examined the effect of MMWs on stress sensitive genes, stress sensitive gene promotors and chaperone proteins in human glial cell lines. In two studies, glial cells were exposed to low-level MMWs and there was no observed modification in the expression of stress sensitive gene promotors when compared with sham exposed cells [ 59 , 60 , 61 ]. Further, glial cells were examined for the expression of the chaperone protein clusterin (CLU) and heat shock protein HSP70. These proteins are activated in times of cellular stress to maintain protein functions and help with the repair process [ 60 ]. There was no observed modification in gene expression of the chaperone proteins. Other studies have examined the endoplasmic reticulum of glial cells exposed to MMWs [ 62 , 63 ]. The endoplasmic reticulum is the site of synthesis and folding of secreted proteins and has been shown to be sensitive to environmental insults [ 62 ]. The authors reported that there was no elevation in mRNA expression levels of endoplasmic reticulum specific chaperone proteins. Studies of stress sensitive genes in glial cells have consistently shown no modification due to low-level MMW exposure [ 59 , 60 , 61 , 62 , 63 ].
Belyaev and co-authors have studied a possible resonance effect of low-level MMWs primarily on Escherichia Coli (E. coli) cells and cultures. The Belyaev research group reported that the resonance effect of MMWs can change the conformation state of chromosomal DNA complexes [ 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ]; however, most of these experiments were not temperature controlled. This resonance effect was not supported by earlier experiments on a number of different cell types conducted by Gandhi et al. and Bush et al. [ 75 , 76 ].
The results of Belyaev and co-workers have primarily been based on evidence from the anomalous viscosity time dependence (AVTD) method [ 77 ]. The research group argued that changes in the AVTD curve can indicate changes to the DNA conformation state and DNA-protein bonds. Belyaev and co-workers have reported in a number of studies that differences in the AVTD curve were dependent on several parameter including MMW characteristics (frequency, exposure level, and polarisation), cellular concentration and cell growth rate [ 69 , 71 , 72 , 73 , 74 ]. In some of the Belyaev studies E. coli were pre-exposed to X-rays, which was reported to change the AVTD curve; however, if the cells were then exposed to MMWs there was no longer a change in the AVTD curve [ 64 , 65 , 66 , 67 ]. The authors suggested that exposure to MMWs increased the rate of recovery in bacterial cells previously exposed to ionising radiation. The Belyaev group also used rat thymocytes in another study and they concluded that the results closely paralleled those found in E. coli cells [ 67 ]. The studies on the DNA conformation state change relied heavily on the AVTD method that has only been used by the Balyaev group and has not been independently validated [ 78 ].
Cell signalling and electrical activity
Studies examining effects of low-level MMWs on cell signalling have mainly involved MMW exposure to nervous system tissue of various animals. An in vivo study on rats recorded extracellular background electrical spike activity from neurons in the supraoptic nucleus of the hypothalamus after MMW exposure [ 79 ]. The study reported that there were changes in inter-spike interval and spike activity in the cells of exposed animals when compared with controls. There was also a mixture of significant shifts in neuron population proportions and spike frequency. The effect on the regularity of neuron spike activity was greater at higher frequencies. An in vitro study on rat cortical tissue slices reported that neuron firing rates decreased in half of the samples exposed to low-level MMWs [ 80 ]. The width of the signals was also decreased but all effects were short lived. The observed changes were not consistent between the two studies, but this could be a consequence of different brain regions being studied.
In vitro experiments by a Japanese research group conducted on crayfish exposed the dissected optical components and brain to MMWs [ 81 , 82 ]. Munemori and Ikeda reported that there was no significant change in the inter-spike intervals or amplitude of spontaneous discharges [ 81 ]. However, there was a change in the distribution of inter-spike intervals where the initial standard deviation decreased and then restored in a short time to a rhythm comparable to the control. A follow-up study on the same tissues and a wide range of exposure levels (many above the ICNIRP limits) reported similar results with the distribution of spike intervals decreasing with increasing exposure level [ 82 ]. These results on action potentials in crayfish tissue have not been independently investigated.
Mixed results were reported in experiments conducted by a US research group on sciatic frog nerve preparations. These studies applied electrical stimulation to the nerve and examined the effect of MMWs on the compound action potentials (CAPs) conductivity through the neurological tissue fibre. Pakhomov et al. found a reduction in CAP latency accompanied by an amplitude increase for MMWs above the ICNIRP limits but not for low-level MMWs [ 83 ]. However, in two follow-up studies, Pakhomov et al. reported that the attenuation in amplitude of test CAPs caused by high-rate stimulus was significantly reduced to the same magnitude at various MMW exposure levels [ 84 , 85 ]. In all of these studies, the observed effect on the CAPs was temporal and reversible, but there were implications of a frequency specific resonance interaction with the nervous tissue. These results on action potentials in frog sciatic nerves have not been investigated by others.
Other common experimental systems involved low-level MMW exposure to isolated ganglia of leeches. Pikov and Siegel reported that there was a decrease in the firing rate in one of the tested neurons and, through the measurement of input resistance in an inserted electrode, there was a transient dose-dependent change in membrane permeability [ 86 ]. However, Romanenko et al. found that low-level MMWs did not cause suppression of neuron firing rate [ 87 ]. Further experiments by Romanenko et al. reported that MMWs at the ICNIRP public exposure limit and above reported similar action potential firing rate suppression [ 88 ]. Significant differences were reported between MMW effects and effects due to an equivalent rise in temperature caused by heating the bathing solution by conventional means.
Membrane effects
Studies examining membrane interactions with low-level MMWs have all been conducted at frequencies above 40 GHz in in vitro experiments. A number of studies investigated membrane phase transitions involving exposure to a range of phospholipid vesicles prepared to mimic biological cell membranes. One group of studies by an Italian research group reported effects on membrane hydration dynamics and phase transition [ 89 , 90 , 91 ]. Observations included transition delays from the gel to liquid phase or vice versa when compared with sham exposures maintained at the same temperature; the effect was reversed after exposure. These reported changes remain unconfirmed by independent groups.
A number of studies investigated membrane permeability. One study focussed on Ca 2+ activated K + channels on the membrane surface of cultured kidney cells of African Green Marmosets [ 92 ]. The study reported modifications to the Hill coefficient and apparent affinity of the Ca 2+ by the K + channels. Another study reported that the effectiveness of a chemical to supress membrane permeability in the gap junction was transiently reduced when the cells were exposed to MMWs [ 93 , 94 ]. Two studies by one research group reported increases in the movement of molecules into skin cells during MMW exposure and suggested this indicates increased cell membrane permeability [ 21 , 91 ]. Permeability changes based on membrane pressure differences were also investigated in relation to phospholipid organisation [ 95 ]. Although there was no evidence of effects on phospholipid organisation on exposed model membranes, the authors reported a measurable difference in membrane pressure at low exposure levels. Another study reported neuron shrinkage and dehydration of brain tissues [ 96 ]. The study reported this was due to influences of low-level MMWs on the cellular bathing medium and intracellular water. Further, the authors suggested this influence of MMWs may have led to formation of unknown messengers, which are able to modulate brain cell hydration. A study using an artificial axon system consisting of a network of cells containing aqueous phospholipid vesicles reported permeability changes with exposure to MMWs by measuring K + efflux [ 97 ]. In this case, the authors emphasised limitations in applying this model to processes within a living organism. The varied effects of low-level MMWs on membrane permeability lack replication.
Other studies have examined the shape or size of vesicles to determine possible effects on membrane permeability. Ramundo-Orlando et al., reported effects on the shape of giant unilamellar vesicles (GUVs), specifically elongation, attributed to permeability changes [ 98 ]. However, another study reported that only smaller diameter vesicles demonstrated a statistically significant change when exposed to MMWs [ 99 ]. A study by Cosentino et al. examined the effect of MMWs on the size distributions of both large unilamellar vesicles (LUVs) and GUVs in in vitro preparations [ 100 ]. It was reported that size distribution was only affected when the vesicles were under osmotic stress, resulting in a statistically significant reduction in their size. In this case, the effect was attributed to dehydration as a result of membrane permeability changes. There is, generally, lack of replication on physical changes to phospholipid vesicles due to low-level MMWs.
Studies on E. coli and E. hirae cultures have reported resonance effects on membrane proteins and phospholipid constituents or within the media suspension [ 39 , 40 , 41 , 42 ]. These studies observed cell proliferation effects such as changes to cell growth rate, viability and lag phase duration. These effects were reported to be more pronounced at specific MMW frequencies. The authors suggested this could be due to a resonance effect on the cell membrane or the suspension medium. Torgomyan et al. and Hovnanyan et al. reported similar changes to proliferation that they attributed to changes in membrane permeability from MMW exposure [ 43 , 45 ]. These experiments were all conducted by an Armenian research group and have not been replicated by others.
Other effects
A number of studies have reported on the experimental results of other effects. Reproductive effects were examined in three studies on mice, rats and human spermatozoa. An in vivo study on mice exposed to low-level MMWs reported that spermatogonial cells had significantly more metaphase translocation disturbances than controls and an increased number of cells with unpaired chromosomes [ 101 ]. Another in vivo study on rats reported increased morphological abnormalities to spermatozoa following exposure, however, there was no statistical analysis presented [ 102 ]. Conversely, an in vitro study on human spermatozoa reported that there was an increase in motility after a short time of exposure to MMWs with no changes in membrane integrity and no generation of apoptosis [ 103 ]. All three of these studies looked at different effects on spermatozoa making it difficult to make an overall conclusion. A further two studies exposed rats to MMWs and examined their sperm for indicators of ROS production. One study reported both increases and decreases in enzymes that control the build-up of ROS [ 104 ]. The other study reported a decrease in the activity of histone kinase and an increase in ROS [ 105 ]. Both studies had low animal numbers (six animals exposed) and these results have not been independently replicated.
Immune function was also examined in a limited number of studies focussing on the effects of low-level MMWs on antigens and antibody systems. Three studies by a Russian research group that exposed neutrophils to MMWs reported frequency dependant changes in ROS production [ 106 , 107 , 108 ]. Another study reported a statistically significant decrease in antigen binding to antibodies when exposed to MMWs [ 109 ]; the study also reported that exposure decreased the stability of previously formed antigen–antibody complexes.
The effect on fatty acid composition in mice exposed to MMWs has been examined by a Russian research group using a number of experimental methods [ 110 , 111 , 112 ]. One study that exposed mice afflicted with an inflammatory condition to low-level MMWs reported no change in the fatty acid concentrations in the blood plasma. However, there was a significant increase in the omega-3 and omega-6 polyunsaturated fatty acid content of the thymus [ 110 ]. Another study exposed tumour-bearing mice and reported that monounsaturated fatty acids decreased and polyunsaturated fatty acids increased in both the thymus and tumour tissue. These changes resulted in fatty acid composition of the thymus tissue more closely resembling that of the healthy control animals [ 111 ]. The authors also examined the effect of exposure to X-rays of healthy mice, which was reported to reduce the total weight of the thymus. However, when the thymus was exposed to MMWs before or after exposure to X-rays, the fatty acid content was restored and was no longer significantly different from controls [ 112 ]. Overall, the authors reported a potential protective effect of MMWs on the recovery of fatty acids, however, all the results came from the same research group with a lack of replication from others.
Physiological effects were examined by a study conducted on mice exposed to WWMs to assess the safety of police radar [ 113 ]. The authors reported no statistically significant changes in the physiological parameters tested, which included body mass and temperature, peripheral blood and the mass and cellular composition, and number of cells in several important organs. Another study exposing human volunteers to low-level MMWs specifically examined cardiovascular function of exposed and sham exposed groups by electrocardiogram (ECG) and atrioventricular conduction velocity derivation [ 114 ]. This study reported that there were no significant differences in the physiological indicators assessed in test subjects.
Other individual studies have looked at various other effects. An early study reported differences in the attenuation of MMWs at specific frequencies in healthy and tumour cells [ 115 ]. Another early study reported no effect in the morphology of BHK-21/C13 cell cultures when exposed to low-level MMWs; the study did report morphological changes at higher levels, which were related to heating [ 116 ]. One study examined whether low-level MMWs induced cancer promotion in leukaemia and Lewis tumour cell grafted mice. The study reported no statistically significant growth promotion in either of the grafted cancer cell types [ 117 ]. Another study looked at the activity of gamma-glutamyl transpeptidase enzyme in rats after treatment with hydrocortisone and exposure to MMWs [ 118 ]. The study reported no effects at exposures below the ICNIRP limit, however, at levels above authors reported a range of effects. Another study exposed saline liquid solutions to continuous low and high level MMWs and reported temperature oscillations within the liquid medium but lacked a statistical analysis [ 119 ]. Another study reported that low-level MMWs decrease the mobility of the protozoa S. ambiguum offspring [ 120 ]. None of the reported effects in all of these other studies have been investigated elsewhere.
Epidemiological studies
There are no epidemiological studies that have directly investigated 5 G and potential health effects. There are however epidemiological studies that have looked at occupational exposure to radar, which could potentially include the frequency range from 6 to 300 GHz. Epidemiological studies on radar were included as they represent occupational exposure below the ICNIRP guidelines. The review included 31 epidemiological studies (8 cohort, 13 case-control, 9 cross-sectional and 1 meta-analysis) that investigated exposure to radar and various health outcomes including cancer at different sites, effects on reproduction and other diseases. The risk estimates as well as limitations of the epidemiological studies are shown in Table 7 .
Three large cohort studies investigated mortality in military personnel with potential exposure to MMWs from radar. Studies reporting on over 40-year follow-up of US navy veterans of the Korean War found that radar exposure had little effect on all-cause or cancer mortality with the second study reporting risk estimates below unity [ 121 , 122 ]. Similarly, in a 40-year follow-up of Belgian military radar operators, there was no statistically significant increase in all-cause mortality [ 123 , 124 ]; the study did, however, find a small increase in cancer mortality. More recently in a 25-year follow-up of military personnel who served in the French Navy, there was no increase in all-cause or cancer mortality for personnel exposed to radar [ 125 ]. The main limitation in the cohort studies was the lack of individual levels of RF exposure with most studies based on job-title. Comparisons were made between occupations with presumed high exposure to RF fields and other occupations with presumed lower exposure. This type of non-differential misclassification in dichotomous exposure assessment is associated mostly with an effect measure biased towards a null effect if there is a true effect of RF fields. If there is no true effect of RF fields, non-differential exposure misclassification will not bias the effect estimate (which will be close to the null value, but may vary because of random error). The military personnel in these studies were compared with the general population and this ‘healthy worker effect’ presents possible bias since military personnel are on average in better health than the general population; the healthy worker effect tends to underestimate the risk. The cohort studies also lacked information on possible confounding factors including other occupational exposures such as chemicals and lifestyle factors such as smoking.
Several epidemiological studies have specifically investigated radar exposure and testicular cancer. In a case-control study where most of the subjects were selected from military hospitals in Washington DC, USA, Hayes et al. found no increased risk between exposure to radar and testicular cancer [ 126 ]; exposure to radar was self-reported and thus subject to misclassification. In this study, the misclassification was likely non-differential, biasing the result towards the null. Davis and Mostofi reported a cluster of testicular cancer within a small cohort of 340 police officers in Washington State (USA) where the cases routinely used handheld traffic radar guns [ 127 ]; however, exposure was not assessed for the full cohort, which may have overestimated the risk. In a population-based case-control study conducted in Sweden, Hardell et al. did not find a statistically significant association between radar work and testicular cancer; however, the result was based on only five radar workers questioning the validity of this result [ 128 ]. In a larger population-based case control study in Germany, Baumgardt-Elms et al. also reported no association between working near radar units (both self-reported and expert assessed) and testicular cancer [ 129 ]; a limitation of this study was the low participation of identified controls (57%), however, there was no difference compared with the characteristics of the cases so selection bias was unlikely. In the cohort study of US navy veterans previously mentioned exposure to radar was not associated with testicular cancer [ 122 ]; the limitations of this cohort study mentioned earlier may have underestimated the risk. Finally, in a hospital-based case-control study in France, radar workers were also not associated with risk of testicular cancer [ 130 ]; a limitation was the low participation of controls (37%) with a difference in education level between participating and non-participating controls, which may have underestimated this result.
A limited number of studies have investigated radar exposure and brain cancer. In a nested case-control study within a cohort of male US Air Force personnel, Grayson reported a small association between brain cancer and RF exposure, which included radar [ 131 ]; no potential confounders were included in the analysis, which may have overestimated the result. However, in a case-control study of personnel in the Brazilian Navy, Santana et al. reported no association between naval occupations likely to be exposed to radar and brain cancer [ 132 ]; the small number of cases and lack of diagnosis confirmation may have biased the results towards the null. All of the cohort studies on military personnel previously mentioned also examined brain cancer mortality and found no association with exposure to radar [ 122 , 124 , 125 ].
A limited number of studies have investigated radar exposure and ocular cancer. Holly et al. in a population-based case-control study in the US reported an association between self-reported exposure to radar or microwaves and uveal melanoma [ 133 ]; the study investigated many different exposures and the result is prone to multiple testing. In another case-control study, which used both hospital and population controls, Stang et al. did not find an association between self-reported exposure to radar and uveal melanoma [ 134 ]; a high non-response in the population controls (52%) and exposure misclassification may have underestimated this result. The cohort studies of the Belgian military and French navy also found no association between exposure to radar and ocular cancer [ 124 , 125 ].
A few other studies have examined the potential association between radar and other cancers. In a hospital-based case-control study in Italy, La Vecchia investigated 14 occupational agents and risk of bladder cancer and found no association with radar, although no risk estimate was reported [ 135 ]; non-differential self-reporting of exposure may have underestimated this finding if there is a true effect. Finkelstein found an increased risk for melanoma in a large cohort of Ontario police officers exposed to traffic radar and followed for 31 years [ 136 ]; there was significant loss to follow up which may have biased this result in either direction. Finkelstein found no statistically significant associations with other types of cancer and the study reported a statistically significant risk estimate just below unity for all cancers, which is reflective of the healthy worker effect [ 136 ]. In a large population-based case-control study in France, Fabbro-Peray et al. investigated a large number of occupational and environmental risk factors in relation to non-Hodgkin lymphoma and found no association with radar operators based on job-title; however, the result was based on a small number of radar operators [ 137 ]. The cohort studies on military personnel did not find statistically significant associations between exposure to radar and other cancers [ 122 , 124 , 125 ].
Variani et al. conducted a recent systematic review and meta-analysis investigating occupational exposure to radar and cancer risk [ 138 ]. The meta-analysis included three cohort studies [ 122 , 124 , 125 ] and three case-control studies [ 129 , 130 , 131 ] for a total sample size of 53,000 subjects. The meta-analysis reported a decrease in cancer risk for workers exposed to radar but noted the small number of studies included with significant heterogeneity between the studies.
Apart from cancer, a number of epidemiological studies have investigated radar exposure and reproductive outcomes. Two early studies on military personnel in the US [ 139 ] and Denmark [ 140 ] reported differences in semen parameters between personnel using radar and personnel on other duty assignments; these studies included only volunteers with potential fertility concerns and are prone to bias. A further volunteer study on US military personnel did not find a difference in semen parameters in a similar comparison [ 141 ]; in general these type of cross-sectional investigations on volunteers provide limited evidence on possible risk. In a case-control study of personnel in the French military, Velez de la Calle et al. reported no association between exposure to radar and male infertility [ 142 ]; non-differential self-reporting of exposure may have underestimated this finding if there is a true effect. In two separate cross-sectional studies of personnel in the Norwegian navy, Baste et al. and Møllerløkken et al. reported an association between exposure to radar and male infertility, but there has been no follow up cohort or case control studies to confirm these results [ 143 , 144 ].
Again considering reproduction, a number of studies investigated pregnancy and offspring outcomes. In a population-based case-control study conducted in the US and Canada, De Roos et al. found no statistically significant association between parental occupational exposure to radar and neuroblastoma in offspring; however, the result was based on a small number of cases and controls exposed to radar [ 145 ]. In another cross-sectional study of the Norwegian navy, Mageroy et al. reported a higher risk of congenital anomalies in the offspring of personnel who were exposed to radar; the study found positive associations with a large number of other chemical and physical exposures, but the study involved multiple comparisons so is prone to over-interpretation [ 146 ]. Finally, a number of pregnancy outcomes were investigated in a cohort study of Norwegian navy personnel enlisted between 1950 and 2004 [ 147 ]. The study reported an increase in perinatal mortality for parental service aboard fast patrol boats during a short period (3 months); exposure to radar was one of many possible exposures when serving on fast patrol boats and the result is prone to multiple testing. No associations were found between long-term exposure and any pregnancy outcomes.
There is limited research investigating exposure to radar and other diseases. In a large case-control study of US military veterans investigating a range of risk factors and amyotrophic lateral sclerosis, Beard et al. did not find a statistically significant association with radar [ 148 ]; the study reported a likely under-ascertainment of non-exposed cases, which may have biased the result away from the null. The cohort studies on military personnel did not find statistically significant associations between exposure to radar and other diseases [ 122 , 124 , 125 ].
A number of observational studies have investigated outcomes measured on volunteers in the laboratory. They are categorised as epidemiological studies because exposure to radar was not based on provocation. These studies investigated genotoxicity [ 149 ], oxidative stress [ 149 ], cognitive effects [ 150 ] and endocrine function [ 151 ]; the studies generally reported positive associations with radar. These volunteer studies did not sample from a defined population and are prone to bias [ 152 ].
The experimental studies investigating exposure to MMWs at levels below the ICNIRP occupational limits have looked at a variety of biological effects. Genotoxicity was mainly examined by using comet assays of exposed cells. This approach has consistently found no evidence of DNA damage in skin cells in well-designed studies. However, animal studies conducted by one research group reported DNA strand breaks and changes in enzymes that control the build-up of ROS, noting that these studies had low animal numbers (six animals exposed); these results have not been independently replicated. Studies have also investigated other indications of genotoxicity including chromosome aberrations, micro-nucleation and spindle disturbances. The methods used to investigate these indicators have generally been rigorous; however, the studies have reported contradictory results. Two studies by a Russian research group have also reported indicators of DNA damage in bacteria, however, these results have not been verified by other investigators.
The studies of the effect of MMWs on cell proliferation primarily focused on bacteria, yeast cells and tumour cells. Studies of bacteria were mainly from an Armenian research group that reported a reduction in the bacterial growth rate of exposed E. coli cells at different MMW frequencies; however, the studies suffered from inadequate dosimetry and temperature control and heating due to high RF energy deposition may have contributed to the results. Other authors have reported no effect of MMWs on E. coli cell growth rate. The results on cell proliferation of yeast exposed to MMWs were also contradictory. An Italian research group that has conducted the majority of the studies on tumour cells reported either a reduction or no change in the proliferation of exposed cells; however, these studies also suffered from inadequate dosimetry and temperature control.
The studies on gene expression mainly examined two different indicators, expression of stress sensitive genes and chaperone proteins and the occurrence of a resonance effect in cells to explain DNA conformation state changes. Most studies reported no effect of low-level MMWs on the expression of stress sensitive genes or chaperone proteins using a range of experimental methods to confirm these results; noting that these studies did not use blinding so experimental bias cannot be excluded from the results. A number of studies from a Russian research group reported a resonance effect of MMWs, which they propose can change the conformation state of chromosomal DNA complexes. Their results relied heavily on the AVTD method for testing changes in the DNA conformation state, however, the biological relevance of results obtained through the AVTD method has not been independently validated.
Studies on cell signalling and electrical activity reported a range of different outcomes including increases or decreases in signal amplitude and changes in signal rhythm, with no consistent effect noting the lack of blinding in most of the studies. Further, temperature contributions could not be eliminated from the studies and in some cases thermal interactions by conventional heating were studied and found to differ from the MMW effects. The results from some studies were based on small sample sizes, some being confined to a single specimen, or by observed effects only occurring in a small number of the samples tested. Overall, the reported electrical activity effects could not be dismissed as being within normal variability. This is indicated by studies reporting the restoration of normal function within a short time during ongoing exposure. In this case there is no implication of an expected negative health outcome.
Studies on membrane effects examined changes in membrane properties and permeability. Some studies observed changes in transitions from liquid to gel phase or vice versa and the authors implied that MMWs influenced cell hydration, however the statistical methods used in these studies were not described so it is difficult to examine the validity of these results. Other studies observing membrane properties in artificial cell suspensions and dissected tissue reported changes in vesicle shape, reduced cell volume and morphological changes although most of these studies suffered from various methodological problems including poor temperature control and no blinding. Experiments on bacteria and yeast were conducted by the same research group reporting changes in membrane permeability, which was attributed to cell proliferation effects, however, the studies suffered from inadequate dosimetry and temperature control. Overall, although there were a variety of membrane bioeffects reported, these have not been independently replicated.
The limited number of studies on a number of other effects from exposure to MMWs below the ICNIRP limits generally reported little to no consistent effects. The single in vivo study on cancer promotion did not find an effect although the study did not include sham controls. Effects on reproduction were contradictory that may have been influenced by opposing objectives of examining adverse health effects or infertility treatment. Further, the only study on human sperm found no effects of low-level MMWs. The studies on reproduction suffered from inadequate dosimetry and temperature control, and since sperm is sensitive to temperature, the effect of heating due to high RF energy deposition may have contributed to the studies showing an effect. A number of studies from two research groups reported effects on ROS production in relation to reproduction and immune function; the in vivo studies had low animal numbers (six animals per exposure) and the in vitro studies generally had inadequate dosimetry and temperature control. Studies on fatty acid composition and physiological indicators did not generally show any effects; poor temperature control was also a problem in the majority of these studies. A number of other studies investigating various other biological effects reported mixed results.
Although a range of bioeffects have been reported in many of the experimental studies, the results were generally not independently reproduced. Approximately half of the studies were from just five laboratories and several studies represented a collaboration between one or more laboratories. The exposure characteristics varied considerably among the different studies with studies showing the highest effect size clustered around a PD of approximately 1 W/m 2 . The meta-analysis of the experimental studies in our companion paper [ 9 ] showed that there was no dose-response relationship between the exposure (either PD or SAR) and the effect size. In fact, studies with a higher exposure tended to show a lower effect size, which is counterfactual. Most of the studies showing a large effect size were conducted in the frequency range around 40–55 GHz, representing investigations into the use of MMWs for therapeutic purposes, rather than deleterious health consequences. Future experimental research would benefit from investigating bioeffects at the specific frequency range of the next stage of the 5 G network roll-out in the range 26–28 GHz. Mobile communications beyond the 5 G network plan to use frequencies higher than 30 GHz so research across the MMW band is relevant.
An investigation into the methods of the experimental studies showed that the majority of studies were lacking in a number of quality criteria including proper attention to dosimetry, incorporating positive controls, using blind evaluation or accurately measuring or controlling the temperature of the biological system being tested. Our meta-analysis showed that the bulk of the studies had a quality score lower than 2 out of a possible 5, with only one study achieving a maximum quality score of 5 [ 9 ]. The meta-analysis further showed that studies with a low quality score were more likely to show a greater effect. Future research should pay careful attention to the experimental design to reduce possible sources of artefact.
The experimental studies included in this review reported PDs below the ICNIRP exposure limits. Many of the authors suggested that the resulting biological effects may be related to non-thermal mechanisms. However, as is shown in our meta-analysis, data from these studies should be treated with caution because the estimated SAR values in many of the studies were much higher than the ICNIRP SAR limits [ 9 ]. SAR values much higher than the ICNIRP guidelines are certainly capable of producing significant temperature rise and are far beyond the levels expected for 5 G telecommunication devices [ 1 ]. Future research into the low-level effects of MMWs should pay particular attention to appropriate temperature control in order to avoid possible heating effects.
Although a systematic review of experimental studies was not conducted, this paper presents a critical appraisal of study design and quality of all available studies into the bioeffects of low level MMWs. The conclusions from the review of experimental studies are supported by a meta-analysis in our companion paper [ 9 ]. Given the low-quality methods of the majority of the experimental studies we infer that a systematic review of different bioeffects is not possible at present. Our review includes recommendations for future experimental research. A search of the available literature showed a further 44 non-English papers that were not included in our review. Although the non-English papers may have some important results it is noted that the majority are from research groups that have published English papers that are included in our review.
The epidemiological studies on MMW exposure from radar that has a similar frequency range to that of 5 G and exposure levels below the ICNIRP occupational limits in most situations, provided little evidence of an association with any adverse health effects. Only a small number of studies reported positive associations with various methodological issues such as risk of bias, confounding and multiple testing questioning the result. The three large cohort studies of military personnel exposed to radar in particular did not generally show an association with cancer or other diseases. A key concern across all the epidemiological studies was the quality of exposure assessment. Various challenges such as variability in complex occupational environments that also include other co-exposures, retrospective estimation of exposure and an appropriate exposure metric remain central in studies of this nature [ 153 ]. Exposure in most of the epidemiological studies was self-reported or based on job-title, which may not necessarily be an adequate proxy for exposure to RF fields above 6 GHz. Some studies improved on exposure assessment by using expert assessment and job-exposure matrices, however, the possibility of exposure misclassification is not eliminated. Another limitation in many of the studies was the poor assessment of possible confounding including other occupational exposures and lifestyle factors. It should also be noted that close proximity to certain very powerful radar units could have exceeded the ICNIRP occupational limits, therefore the reported effects especially related to reproductive outcomes could potentially be related to heating.
Given that wireless communications have only recently started to use RF frequencies above 6 GHz there are no epidemiological studies investigating 5 G directly as yet. Some previous epidemiological studies have reported a possible weak association between mobile phone use (from older networks using frequencies below 6 GHz) and brain cancer [ 11 ]. However, methodological limitations in these studies prevent conclusions of causality being drawn from the observations [ 152 ]. Recent investigations have not shown an increase in the incidence of brain cancer in the population that can be attributed to mobile phone use [ 154 , 155 ]. Future epidemiological research should continue to monitor long-term health effects in the population related to wireless telecommunications.
The review of experimental studies provided no confirmed evidence that low-level MMWs are associated with biological effects relevant to human health. Many of the studies reporting effects came from the same research groups and the results have not been independently reproduced. The majority of the studies employed low quality methods of exposure assessment and control so the possibility of experimental artefact cannot be excluded. Further, many of the effects reported may have been related to heating from high RF energy deposition so the assertion of a ‘low-level’ effect is questionable in many of the studies. Future studies into the low-level effects of MMWs should improve the experimental design with particular attention to dosimetry and temperature control. The results from epidemiological studies presented little evidence of an association between low-level MMWs and any adverse health effects. Future epidemiological research would benefit from specific investigation on the impact of 5 G and future telecommunication technologies.
Wu T, Rappaport TS, Collins CM. Safe for generations to come: considerations of safety for millimeter waves in wireless communications. IEEE Micro Mag. 2015;16:65–84.
Article Google Scholar
Health protection agency (HPA). Health effects from radiofrequency electromagnetic fields: the report of the independent advisory group on non-ionising radiation (AGNIR). HPA. 2012; RCE 20.
Scientific committee on emerging and newly identified health risks (SCENHIR). Potential health effects of exposure to electromagnetic fields (EMF). Euro Comm. 2015; 1831-4783.
Australian radiation protection and nuclear safety agency (ARPANSA). Radiation protection standard for maximum exposure levels to radiofrequency fields—3 kHz to 300 GHz. Radiation Protection Series 3. ARPANSA; 2002.
International Commission on Non-Ionizing Radiation Protection (ICNIRP). ICNIRP guidelines for limiting exposure to electromagnetic fields (100 KHz to 300 GHz). Health Phys. 2020;118:483–524.
Article CAS PubMed Google Scholar
Institute of electrical and electronics engineers (IEEE). IEEE standard for safety levels with respect to human exposure to electric, magnetic, and electromagnetic fields, 0 Hz to 300 GHz. IEEE 2019; C95.1.
Stam R. Comparison of international policies on electromagnetic fields (power frequency and radiofrequency fields). National institute for public health and the environment, RIVM 2018.
Simkó M, Mattsson MO. 5G Wireless communication and health effects—a pragmatic review based on available studies regarding 6 to 100 GHz. Int J Environ Res Public Health. 2019;16:3406.
Article PubMed Central CAS Google Scholar
Wood A, Mate R, Karipidis K. Meta-analysis of in vitro and in vivo studies of the biological effects of low-level millimetre waves. 2020. https://doi.org/10.1038/s41370-021-00307-7 .
International commission on non-Ionizing radiation protection (ICNIRP). Exposure to high frequency electromagnetic fields, biological effects and health consequences (100 kHz-300 GHz). ICNIRP 2009; 978-3-934994-10-2.
International agency for research on cancer (IARC). IARC monographs: non-ionizing radiation, part 2: radiofrequency electromagnetic fields. IARC 2013;102:1–460.
Google Scholar
Garaj-Vrhovac V, Horvat D, Koren Z. The relationship between colony-forming ability, chromosome aberrations and incidence of micronuclei in V79 Chinese hamster cells exposed to microwave radiation. Mutat Res Lett. 1991;263:143–9.
Article CAS Google Scholar
Garaj-Vrhovac V, Fučić A, Horvat D. The correlation between the frequency of micronuclei and specific chromosome aberrations in human lymphocytes exposed to microwave radiation in vitro. Mutat Res Lett. 1992;281:181–6.
Korenstein-Ilan A, Barbul A, Hasin P, Eliran A, Gover A, Korenstein R. Terahertz radiation increases genomic instability in human lymphocytes. Radiat Res. 2008;170:224–34.
Hintzsche H, Jastrow C, Kleine-Ostmann T, Kärst U, Schrader T, Stopper H. Terahertz electromagnetic fields (0.106 THz) do not induce manifest genomic damage in vitro. PloS One. 2012;7:e46397.
Koyama S, Narita E, Shimizu Y, Suzuki Y, Shiina T, Taki M, et al. Effects of long-term exposure to 60 GHz millimeter-wavelength radiation on the genotoxicity and heat shock protein (Hsp) expression of cells derived from human eye. Int J Environ Res Public Health. 2016;13:802.
Koyama S, Narita E, Suzuki Y, Shiina T, Taki M, Shinohara N, et al. Long-term exposure to a 40-GHz electromagnetic field does not affect genotoxicity or heat shock protein expression in HCE-T or SRA01/04 cells. J Radiat Res. 2019;60:417–23.
Article CAS PubMed PubMed Central Google Scholar
De Amicis A, De Sanctis S, Di Cristofaro S, Franchini V, Lista F, Regalbuto E, et al. Biological effects of in vitro THz radiation exposure in human foetal fibroblasts. Mutat Res Genet Toxicol Environ Mutagen. 2015;793:150–60.
Franchini V, Regalbuto E, De Amicis A, De Sanctis S, Di Cristofaro S, Coluzzi E, et al. Genotoxic effects in human fibroblasts exposed to microwave radiation. Health Phys. 2018;115:126–39.
Shckorbatov YG, Grigoryeva NN, Shakhbazov VG, Grabina VA, Bogoslavsky AM. Microwave irradiation influences on the state of human cell nuclei. Bioelectromagnetics. 1998;19:414–9.
Shckorbatov YG, Pasiuga VN, Kolchigin NN, Grabina VA, Batrakov DO, Kalashnikov VV. The influence of differently polarised microwave radiation on chromatin in human cells. Int J Radiat Biol. 2009;85:322–9.
Shckorbatov YG, Pasiuga VN, Goncharuk EI, Petrenko TP, Grabina VA, Kolchigin NN, et al. Effects of differently polarized microwave radiation on the microscopic structure of the nuclei in human fibroblasts. J Zhejiang Univ Sci B. 2010;11:801–5.
Article PubMed PubMed Central Google Scholar
Paulraj R, Behari J. Single strand DNA breaks in rat brain cells exposed to microwave radiation. Mutat Res. 2006;596:76–80.
Kesari KK, Behari J. Fifty-gigahertz microwave exposure effect of radiations on rat brain. Appl Biochem Biotechnol. 2009;158:126.
Kumar S, Kesari KK, Behari J. Evaluation of genotoxic effects in male Wistar rats following microwave exposure. Indian J Exp Biol. 2010;48:586–92.
PubMed Google Scholar
Crouzier D, Perrin A, Torres G, Dabouis V, Debouzy JC. Pulsed electromagnetic field at 9.71 GHz increase free radical production in yeast (Saccharomyces cerevisiae). Patho Biol. 2009;57:245–51.
Smolyanskaya AZ, Vilenskaya RL. Effects of millimeter-band electromagnetic radiation on the functional activity of certain genetic elements of bacterial cells. Sov Phys. 1974;16:571. USPEKHI
Lukashevsky KV, Belyaev IY. Switching of prophage lambda genes in Escherichia coli by millimetre waves. Med Sci Res. 1990;18:955–7.
Kalantaryan VP, Vardevanyan PO, Babayan YS, Gevorgyan ES, Hakobyan SN, Antonyan AP. Influence of low intensity coherent electromagnetic millimeter radiation (EMR) on aqua solution of DNA. Prog Electromag Res. 2010;13:1–9.
Hintzsche H, Jastrow C, Kleine-Ostmann T. Terahertz radiation induces spindle disturbances in human-hamster hybrid cells. Radiat Res. 2011;175:569–74.
Zeni O, Gallerano GP, Perrotta A, Romano M, Sannino A, Sarti M, et al. Cytogenetic observations in human peripheral blood leukocytes following in vitro exposure to THz radiation: a pilot study. Health Phys. 2007;92:349–57.
Gapeyev A, Lukyanova N, Gudkov S. Hydrogen peroxide induced by modulated electromagnetic radiation protects the cells from DNA damage. Open Life Sci. 2014;9:915–21.
Gapeyev AB, Lukyanova NA. Pulse-modulated extremely high-frequency electromagnetic radiation protects cellular DNA from the damaging effects of physical and chemical factors in vitro. Biophys. 2015;60:732–8.
Webb SJ, Dodds DD. Inhibition of bacterial cell growth by 136 GC microwaves. Nature. 1968;218:374–5.
Webb SJ, Booth AD. Absorption of microwaves by microorganisms. Nature. 1969;222:1199–200.
Rojavin MA, Ziskin MC. Effect of millimeter waves on survival of UVC‐exposed Escherichia coli. Bioelectromagnetics. 1995;16:188–96.
Pakhomova ON, Pakhomov AG, Akyel Y. Effect of millimeter waves on UV-induced recombination and mutagenesis in yeast. Bioelectrochem Bioenerg. 1997;43:227–32.
Cohen I, Cahan R, Shani G, Cohen E, Abramovich A. Effect of 99 GHz continuous millimeter wave electro-magnetic radiation on E. coli viability and metabolic activity. Int J Radiat Biol. 2010;86:390–9.
Tadevosyan H, Kalantaryan V, Trchounian A. Extremely high frequency electromagnetic radiation enforces bacterial effects of inhibitors and antibiotics. Cell Biochem Biophys. 2008;51:97–103.
Torgomyan H, Trchounian A. Low-intensity electromagnetic irradiation of 70.6 and 73 GHz frequencies enhances the effects of disulfide bonds reducer on Escherichia coli growth and affects the bacterial surface oxidation–reduction state. Biochem Biophys Res Commun. 2011;414:265–9.
Torgomyan H, Kalantaryan V, Trchounian A. Low intensity electromagnetic irradiation with 70.6 and 73 GHz frequencies affects Escherichia coli growth and changes water properties. Cell Biochem Biophys. 2011;60:275–81.
Torgomyan H, Hovnanyan K, Trchounian A. Escherichia coli growth changes by the mediated effects after low-intensity electromagnetic irradiation of extremely high frequencies. Cell Biochem Biophys. 2012;65:445–54.
Torgomyan H, Ohanyan V, Blbulyan S, Kalantaryan V, Trchounian A. Electromagnetic irradiation of Enterococcus hirae at low-intensity 51.8-and 53.0-GHz frequencies: changes in bacterial cell membrane properties and enhanced antibiotics effects. FEMS microbiol Lett. 2012;329:131–7.
Soghomonyan D, Trchounian A. Comparable effects of low-intensity electromagnetic irradiation at the frequency of 51.8 and 53 GHz and antibiotic ceftazidime on Lactobacillus acidophilus growth and survival. Cell Biochem Biophys. 2013;67:829–35.
Hovnanyan K, Kalantaryan V, Trchounian A. The distinguishing effects of low‐intensity electromagnetic radiation of different extremely high frequencies on Enterococcus hirae: growth rate inhibition and scanning electron microscopy analysis. Lett Appl microbiol. 2017;65:220–5.
Grundler W, Keilmann F. Nonthermal effects of millimeter microwaves on yeast growth. Z Naturforsch. 1977;33:15–22.
Grundler W, Keilmann F. Sharp resonances in yeast growth prove nonthermal sensitivity to microwaves. Phys Rev Lett. 1983;51:1214.
Furia L, Hill DW, Gandhi OMP. Effect of millimeter-wave irradiation on growth of Saccharomyces cerevisiae. IEEE Trans Biom Eng. 1986;33:993–9.
Gos P, Eicher B, Kohli J, Heyer WD. Extremely high frequency electromagnetic fields at low power density do not affect the division of exponential phase Saccharomyces cerevisiae cells. Bioelectromagnetics. 1997;18:142–55.
Chidichimo G, Beneduci A, Nicoletta M, Critelli M, De RR, Tkatchenko Y, et al. Selective inhibition of tumoral cells growth by low power millimeter waves. Anticancer Res. 2002;22:1681–8.
Beneduci A, Chidichimo G, Tripepi S, Perrotte E. Frequency and irradiation time-dependant antiproliferative effect of low-power millimeter waves on RPMI 7932 human melanoma cell line. Anticancer Res. 2005;25(2A):1023–8.
Beneduci A, Chidichimo G, Tripepi S, Perrotte E. Transmission electron microscopy study of the effects produced by wide-band low-power millimeter waves on MCF-7 human breast cancer cells in culture. Anticancer Res. 2005;25(2A):1009–13.
Beneduci A. Evaluation of the potential in vitro antiproliferative effects of millimeter waves at some therapeutic frequencies on RPMI 7932 human skin malignant melanoma cells. Cell Biochem Biophys. 2009;1:25–32.
Beneduci A, Chidichimo G, Tripepi S, Perrotta E, Cufone F. Antiproliferative effect of millimeter radiation on human erythromyeloid leukemia cell line K562 in culture: ultrastructural-and metabolic-induced changes. Bioelectrochemistry. 2007;70:214–20.
Yaekashiwa N, Otsuki S, Hayashi SI, Kawase K. Investigation of the non-thermal effects of exposing cells to 70–300 GHz irradiation using a widely tunable source. J Radiat Res. 2017;59:116–21.
Badzhinyan SA, Sayadyan AB, Sarkisyan NK, Grigoryan RM, Gasparyan GG. Lethal effect of electromagnetic radiation of the millimeter wavelength range on cell cultures of chicken embryo. Dokl Biochem Biophys. 2001;377:94–5.
Shiina T, Suzuki Y, Kasai Y, Inami Y, Taki M, Wake K. Effect of two-times 24 h exposures to 60 GHz millimeter-waves on neurite outgrowth in PC12VG cells in consideration of polarization. IEEE Int Sympo Electromag Compat. 2014;13:166–9.
Le Quément C, Nicolas Nicolaz C, Zhadobov M, Desmots F, Sauleau R, Aubry M, et al. Whole‐genome expression analysis in primary human keratinocyte cell cultures exposed to 60 GHz radiation. Bioelectromagnetics. 2012;33:147–58.
Article PubMed CAS Google Scholar
Zhadobov M, Sauleau R, Le Coq L, Thouroude D, Orlov I, Michel D et al. 60 GHz electromagnetic fields do not activate stress-sensitive gene expression. IEEE 11th Int Sympo on Antenna Technol and appl electromag. 2005;11:1–4.
Zhadobov M, Sauleau R, Le Coq L, Debure L, Thouroude D, Michel D, et al. Low‐power millimeter wave radiations do not alter stress‐sensitive gene expression of chaperone proteins. Bioelectromagnetics. 2007;28:188–96.
Zhadobov M, Nicolaz CN, Sauleau R, Desmots F, Thouroude D, Michel D, et al. Evaluation of the potential biological effects of the 60-GHz millimeter waves upon human cells. IEEE Trans Antennas Propag. 2009;57:2949–56.
Nicolaz CN, Zhadobov M, Desmots F, Ansart A, Sauleau R, Thouroude D, et al. Study of narrow band millimeter‐wave potential interactions with endoplasmic reticulum stress sensor genes. Bioelectromagnetics. 2008;30:365–73.
Nicolaz CN, Zhadobov M, Desmots F, Sauleau R, Thouroude D, Michel D, et al. Absence of direct effect of low-power millimeter-wave radiation at 60.4 GHz on endoplasmic reticulum stress. Cell Biol Toxicol. 2009;25:471–8.
Belyaev IY, Alipov YD, Shcheglov VS, Lystsov VN. Resonance effect of microwaves on the genome conformational state of E. coli cells. Z Naturforsch C. 1992;47:621–7.
Belyaev IY, Shcheglov VS, Alipov YD. Existence of selection rules on helicity during discrete transitions of the genome conformational state of E. coli cells exposed to low-level millimetre radiation. Bioelectrochem Bioenerg. 1992;27:405–11.
Belyaev IY, Shcheglov VS, Alipov YD. Selection rules on helicity during discrete transitions of the genome conformational state in intact and X-rayed cells of E. coli in millimeter range of electromagnetic field. Charg Field Eff Biosyst. 1992;3:115–26.
Belyaev I, Alipov YD, Shcheglov VS, Chromosome DNA. as a target of resonant interaction between Escherichia coli cells and low–intensity millimeter waves. Electro Magnetobiol. 1992;11:97–108.
Belyaev IY, Alipov YD, Polunin VA, Shcheglov VS. Evidence for dependence of resonant frequency of millimeter wave interaction with Escherichia coli K12 cells on haploid genome length. Electro Magnetobiol. 1993;12:39–49.
Belyaev IY, Shcheglov VS, Alipov YD, Radko SP. Regularities of separate and combined effects of circularly polarized millimeter waves on E. coli cells at different phases of culture growth. Bioelectrochem Bioenerg. 1993;31:49–63.
Belyaev IY, Alipov YD, Shcheglov VS, Polunin VA, Aizenberg OA. Cooperative response of Escherichia coli cells to the resonance effect of millimeter waves at super low intensity. Electro Magnetobiol. 1994;13:53–66.
Belyaev IY, Kravchenko VG. Resonance effect of low-intensity millimeter waves on the chromatin conformational state of rat thymocytes. Z Naturforsch. 1994;49:352–8.
Belyaev IY, Shcheglov VS, Alipov YD, Polunin VA. Resonance effect of millimeter waves in the power range from 10‐19 to 3× 10‐3 W/cm2 on Escherichia coli cells at different concentrations. Bioelectromagnetics. 1996;17:312–21.
Shcheglov VS, Belyaev I, Alipov YD, Ushakov VL. Power-dependent rearrangement in the spectrum of resonance effect of millimeter waves on the genome conformational state of Escherichia Coli cells. Electro Magnetobiol. 1997;16:69–82.
Shcheglov VS, Alipov ED, Belyaev I. Cell-to-cell communication in response of E. coli cells at different phases of growth to low-intensity microwaves. Biochim biophys Acta. 2002;1572:101–6.
Gandhi OP, Hagmann MJ, Hill DW, Partlow LM, Bush L. Millimeter wave absorption spectra of biological samples. Bioelectromagnetics. 1980;1:285–98.
Bush LG, Hill DW, Riazi A, Stensaas LJ, Partlow LM, Gandhi OP. Effects of millimeter‐wave radiation on monolayer cell cultures. III. A search for frequency‐specific athermal biological effects on protein synthesis. Bioelectromagnetics. 1981;2:151–9.
Belyaev IY, Shcheglov VS, Alipov ED, Ushakov VD. Nonthermal effects of extremely high-frequency microwaves on chromatin conformation in cells in vitro—dependence on physical, physiological, and genetic factors. IEEE Trans Micro Theory Tech. 2000;48:2172–9.
Pakhomov AG, Akyel Y, Pakhomova ON, Stuck BE, Murphy MR. Current state and implications of research on biological effects of millimeter waves: a review of the literature. Bioelectromagnetics. 1998;19:393–413.
Minasyan SM, Grigoryan GY, Saakyan SG, Akhumyan AA, Kalantaryan VP. Effects of the action of microwave-frequency electromagnetic radiation on the spike activity of neurons in the supraoptic nucleus of the hypothalamus in rats. Neurosci Behav Physiol. 2007;37:175–80.
Pikov V, Arakaki X, Harrington M, Fraser SE, Siegel PH. Modulation of neuronal activity and plasma membrane properties with low-power millimeter waves in organotypic cortical slices. J Neural Eng. 2010;7:045003.
Article PubMed Google Scholar
Munemori J, Ikeda T. Effects of low-level microwave radiation on the eye of the crayfish. Med Biol Eng Comput. 1982;20:84–8.
Munemori J, Ikeda T. Biological effects of X-band microwave radiation on the eye of the crayfish. Med Biol Eng Comput. 1984;22:263–7.
Pakhomov AG, Prol HK, Mathur SP, Akyel Y, Campbell CB. Frequency-specific effects of millimeter-wavelength electromagnetic radiation in isolated nerve. Electro Magnetobiol. 1997;16:43–57.
Pakhomov AG, Prol HK, Mathur SP, Akyel Y, Campbell CB. Search for frequency‐specific effects of millimeter‐wave radiation on isolated nerve function. Bioelectromagnetics. 1997;18:324–34.
Pakhomov AG, Prol HK, Mathur SP, Akyel Y, Campbell CB. Role of field intensity in the biological effectiveness of millimeter waves at a resonance frequency. Bioelectrochem Bioenerg. 1997;43:27–33.
Pikov V, Siegel PH. Millimeter wave-induced changes in membrane properties of leech Retzius neurons. Photonic Therapeutics Diagnostics. 2011;7883:56–1.
Romanenko S, Siegel PH, Pikov V. Microdosimetry and physiological effects of millimeter wave irradiation in isolated neural ganglion preparation. IEEE 2013 International kharkov symposium on physics and engineering of microwaves, millimeter and submillimeter waves. IEEE. 2013;13:512–6.
Romanenko S, Siegel PH, Wagenaar DA, Pikov V. Effects of millimeter wave irradiation and equivalent thermal heating on the activity of individual neurons in the leech ganglion. J Neurophysiol. 2014;112:2423–31.
Beneduci A, Filippelli L, Cosentino K, Calabrese ML, Massa R, Chidichimo G. Microwave induced shift of the main phase transition in phosphatidylcholine membranes. Bioelectrochemistry. 2012;1:18–24.
Beneduci A, Cosentino K, Chidichimo G. Millimeter wave radiations affect membrane hydration in phosphatidylcholine vesicles. Materials. 2013;6:2701–12.
Beneduci A, Cosentino K, Romeo S, Massa R, Chidichimo G. Effect of millimetre waves on phosphatidylcholine membrane models: a non-thermal mechanism of interaction. Soft Matter. 2014;10:5559–67.
Geletyuk VI, Kazachenko VN, Chemeris NK, Fesenko EE. Dual effects of microwaves on single Ca2+-activated K+ channels in cultured kidney cells Vero. FEBS Lett. 1995;359:85–8.
Chen Q, Zeng QL, Lu DQ, Chiang H. Millimeter wave exposure reverses TPA suppression of gap junction intercellular communication in HaCaT human keratinocytes. Bioelectromagnetics. 2004;25:1–4.
Shckorbatov YG, Shakhbazov VG, Navrotskaya VV, Grabina VA, Sirenko SP, Fisun AI, et al. Application of intracellular microelectrophoresis to analysis of the influence of the low‐level microwave radiation on electrokinetic properties of nuclei in human epithelial cells. Electrophoresis. 2002;23:2074–9.
Zhadobov M, Sauleau R, Vié V, Himdi M, Le Coq L, Thouroude D. Interactions between 60-GHz millimeter waves and artificial biological membranes: dependence on radiation parameters. IEEE Trans Micro Theory Tech. 2006;54:2534–42.
Deghoyan A, Heqimyan A, Nikoghosyan A, Dadasyan E, Ayrapetyan S. Cell bathing medium as a target for non thermal effect of millimeter waves. Electromag Biol Med. 2012;31:132–42.
D’Agostino S, Della Monica C, Palizzi E, Di Pietrantonio F, Benetti M, Cannatà D, et al. Extremely high frequency electromagnetic fields facilitate electrical signal propagation by increasing transmembrane potassium efflux in an artificial axon model. Sci Rep. 2018;8:9299.
Article PubMed PubMed Central CAS Google Scholar
Ramundo-Orlando A, Longo G, Cappelli M, Girasole M, Tarricone L, Beneduci A, et al. The response of giant phospholipid vesicles to millimeter waves radiation. Biochem Biophys Acta. 2009;1788:1497–507.
Di Donato L, Cataldo M, Stano P, Massa R, Ramundo-Orlando A. Permeability changes of cationic liposomes loaded with carbonic anhydrase induced by millimeter waves radiation. Radiat Res. 2012;178:437–46.
Cosentino K, Beneduci A, Ramundo-Orlando A, Chidichimo G. The influence of millimeter waves on the physical properties of large and giant unilamellar vesicles. J Biol Phys. 2013;39:395–410.
Manikowska E, Luciani JM, Servantie B, Czerski P, Obrenovitch J, Stahl A. Effects of 9.4 GHz microwave exposure on meiosis in mice. Experientia. 1979;35:388–90.
Subbotina TI, Tereshkina OV, Khadartsev AA, Yashin AA. Effect of low-intensity extremely high frequency radiation on reproductive function in Wistar rats. Bull Exp Biol Med. 2006;142:189–90.
Volkova NA, Pavlovich EV, Gapon AA, Nikolov OT. Effects of millimeter-wave electromagnetic exposure on the morphology and function of human cryopreserved spermatozoa. Bull Exp Biol Med. 2014;157:574–6.
Kesari KK, Behari J. Microwave exposure affecting reproductive system in male rats. Appl Biochem Biotechnol. 2010;162:416–28.
Kumar S, Kesari KK, Behari J. Influence of microwave exposure on fertility of male rats. Fertil Steril. 2011;95:1500–2.
Gapeyev AB, Safronova VG, Chemeris NK, Fesenko EE. Inhibition of the production of reactive oxygen species in mouse peritoneal neutrophils by millimeter wave radiation in the near and far field zones of the radiator. Bioelectrochem Bioenerg. 1997;43:217–20.
Gapeyev AB, Yakushina VS, Chemeris NK, Fesenko EE. Modification of production of reactive oxygen species in mouse peritoneal neutrophils on exposure to low-intensity modulated millimeter wave radiation. Bioelectrochem Bioenerg. 1998;46:267–72.
Safronova VG, Gabdoulkhakova AG, Santalov BF. Immunomodulating action of low intensity millimeter waves on primed neutrophils. Bioelectromagnetics. 2002;23:599–606.
Homenko A, Kapilevich B, Kornstein R, Firer MA. Effects of 100 GHz radiation on alkaline phosphatase activity and antigen–antibody interaction. Bioelectromagnetics. 2009;30:167–75.
Gapeyev AB, Kulagina TP, Aripovsky AV, Chemeris NK. The role of fatty acids in anti‐inflammatory effects of low‐intensity extremely high‐frequency electromagnetic radiation. Bioelectromagnetics. 2011;32:388–95.
Gapeyev AB, Kulagina TP, Aripovsky AV. Exposure of tumor-bearing mice to extremely high-frequency electromagnetic radiation modifies the composition of fatty acids in thymocytes and tumor tissue. Int J Radiat Biol. 2013;89:602–10.
Gapeyev AB, Aripovsky AV, Kulagina TP. Modifying effects of low-intensity extremely high-frequency electromagnetic radiation on content and composition of fatty acids in thymus of mice exposed to X-rays. Int J Radiat Biol. 2015;91:277–85.
Rotkovská D, Moc J, Kautská J, Bartonícková A, Keprtová J, Hofer M. Evaluation of the biological effects of police radar RAMER 7F. Environ Health Perspect. 1993;101:134–6.
PubMed PubMed Central Google Scholar
Müller J, Hadeler KP, Müller V, Waldmann J, Landstorfer FM, Wisniewski R, et al. Influence of low power cm-/mm-microwaves on cardiovascular function. Int J Environ Health Res. 2004;14:331–41.
Webb SJ, Booth AD. Microwave absorption by normal and tumor cells. Science. 1971;1:72–4. 174
Stensaas LJ, Partlow LM, Bush LG, Iversen PL, Hill DW, Hagmann MJ, et al. Effects of millimeter‐wave radiation on monolayer cell cultures. II. Scanning and transmission electron microscopy. Bioelectromagnetics. 1981;2:141–50.
Bellossi A, Dubost G, Moulinoux JP, Himdi M, Ruelloux M, Rocher C. Biological effects of millimeter wave irradiation on mice-preliminary results. IEEE Trans Micro Theory Tech. 2000;48:2104–10.
Olchowik G, Maj JG. Inhibitory action of microwave radiation on gamma-glutamyl transpeptidase activity in liver of rats treated with hydrocortisone. Folia Histochemica Et Cytobiologica. 2000;38:189–91.
CAS PubMed Google Scholar
Khizhnyak EP, Ziskin MC. Temperature oscillations in liquid media caused by continuous (nonmodulated) millimeter wavelength electromagnetic irradiation. Bioelectromagnetics. 1996;17:223–9.
Sarapultseva EI, Igolkina JV, Tikhonov VN, Dubrova YE. The in vivo effects of low-intensity radiofrequency fields on the motor activity of protozoa. Int J Radiat Biol. 2014;90:262–7.
Robinette CD, Silverman C, Jablon S. Effects upon health of occupational exposure to microwave radiation (radar). Am J Epidemiol. 1980;112:39–53.
Groves FD, Page WF, Gridley G, Lisimaque L, Stewart PA, Tarone RE, et al. Cancer in Korean war navy technicians: mortality survey after 40 years. Am J Epidemiol. 2002;155:810–8.
Degrave E, Autier P, Grivegnée AR, Zizi M. All-cause mortality among Belgian military radar operators: a 40-year controlled longitudinal study. Eur J Epidemiol. 2005;20:677–81.
Degrave E, Meeusen B, Grivegnée AR, Boniol M, Autier P. Causes of death among Belgian professional military radar operators: a 37‐year retrospective cohort study. Int J Cancer. 2009;124:945–51.
Dabouis V, Arvers P, Debouzy JC, Sebbah C, Crouzier D, Perrin A. First epidemiological study on occupational radar exposure in the French Navy: a 26-year cohort study. Int J Environ Health Res. 2016;26:131–44.
Hayes RB, Brown LM, Pottern LM, Gomez M, Kardaun JW, Hoover RN, et al. Occupation and risk for testicular cancer: a case-control study. Int J Epidemiol. 1990;19:825–31.
Davis RL, Mostofi FK. Cluster of testicular cancer in police officers exposed to hand‐held radar. Am J Ind Med. 1993;24:231–3.
Hardell LE, Näsman A, Ohlson CG, Fredrikson MA. Case-control study on risk factors for testicular cancer. Int J Oncol. 1998;13:1299–602.
Baumgardt-Elms C, Ahrens W, Bromen K, Boikat U, Stang A, Jahn I, et al. Testicular cancer and electromagnetic fields (EMF) in the workplace: results of a population-based case–control study in Germany. Cancer Causes Control 2002;13:895–902.
Walschaerts M, Muller A, Auger J, Bujan L, Guérin JF, Lannou DL, et al. Environmental, occupational and familial risks for testicular cancer: a hospital‐based case‐control study. Int J Androl. 2007;30:222–9.
Grayson JK. Radiation exposure, socioeconomic status, and brain tumor risk in the US Air Force: a nested case-control study. Am J Epidemiol. 1996;143:480–6.
Santana VS, Silva M, Loomis D. Brain neoplasms among naval military men. Int J Occup Environ health. 1999;5:88–94.
Holly EA, Aston DA, Ahn DK, Smith AH. Intraocular melanoma linked to occupations and chemical exposures. Epidemiology. 1996;1:55–61.
Stang A, Anastassiou G, Ahrens W, Bromen K, Bornfeld N, Jöckel KH. The possible role of radiofrequency radiation in the development of uveal melanoma. Epidemiology. 2001;1:7–12.
La Vecchia CA, Negri E, D’avanzo BA, Franceschi S. Occupation and the risk of bladder cancer. Int J Epidemiol. 1990;19:264–8.
Finkelstein MM. Cancer incidence among Ontario police officers. Am J Ind Med. 1998;34:157–62.
Fabbro-Peray P, Daures JP, Rossi JF. Environmental risk factors for non-Hodgkin’s lymphoma: a population-based case–control study in Languedoc-Roussillon, France. Cancer Causes Control. 2001;12:201–12.
Variani AS, Saboori S, Shahsavari S, Yari S, Zaroushani V. Effect of occupational exposure to radar radiation on cancer risk: a systematic review and meta-analysis. Asian Pac J cancer prev. 2019;20:3211–9.
Weyandt TB, Schrader SM, Turner TW, Simon SD. Semen analysis of military personnel associated with military duty assignments. Reprod Toxicol. 1996;10:521–8.
Hjollund NH, Bonde JP, Skotte J. Semen analysis of personnel operating military radar equipment. Reprod Toxicol. 1997;11:897
Schrader SM, Langford RE, Turner TW, Breitenstein MJ, Clark JC, Jenkins BL. Reproductive function in relation to duty assignments among military personnel. Reprod Toxicol. 1998;12:465–8.
Velez De La Calle JF, Rachou E, le Martelot MT, Ducot B, Multigner L, Thonneau PF. Male infertility risk factors in a French military population. Hum reprod. 2001;16:481–6.
Baste V, Riise T, Moen BE. Radiofrequency electromagnetic fields; male infertility and sex ratio of offspring. Eur J Epidemiol. 2008;23:369–77.
Møllerløkken OJ, Moen BE. Is fertility reduced among men exposed to radiofrequency fields in the Norwegian Navy? Bioelectromagnetics. 2008;29:345–52.
De Roos AJ, Teschke K, Savitz DA, Poole C, Grufferman S, Pollock BH, et al. Parental occupational exposures to electromagnetic fields and radiation and the incidence of neuroblastoma in offspring. Epidemiology. 2001;1:508–17.
Mageroy N, Mollerlokken OJ, Riise T, Koefoed V, Moen BE. A higher risk of congenital anomalies in the offspring of personnel who served aboard a Norwegian missile torpedo boat. Occup Environ Med. 2006;63:92–7.
Baste V, Moen BE, Oftedal G, Strand LA, Bjørge L, Mild KH. Pregnancy outcomes after paternal radiofrequency field exposure aboard fast patrol boats. J Occup Environ Med. 2012;54:431–8.
Beard JD, Kamel F. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis etiology and survival. Epidemiol Rev. 2015;37:55–70.
Garaj-Vrhovac V, Gajski G, Pažanin S, Šarolić A, Domijan AM, Flajs D, et al. Assessment of cytogenetic damage and oxidative stress in personnel occupationally exposed to the pulsed microwave radiation of marine radar equipment. Int J Hyg Environ Health. 2011;214:59–65.
Mortazavi SM, Shahram TA, Dehghan N. Alterations of visual reaction time and short term memory in military radar personnel. Iran J Public Health. 2013;42:428.
Singh S, Mani KV, Kapoor N. Effect of occupational EMF exposure from radar at two different frequency bands on plasma melatonin and serotonin levels. Int J Radiat Biol. 2015;91:426–34.
Ahlbom A, Green A, Kheifets L, Savitz D, Swerdlow A. ICNIRP standing committee on epidemiology: epidemiology of health effects of radiofrequency exposure. Environ Health Perspect. 2004;112:1741–54.
Savitz DA. Exposure assessment strategies in epidemiological studies of health effects of electric and magnetic fields. Sci Total Environ. 1995;168:143–53.
J‐H Kim S, Ioannides SJ, Elwood JM. Trends in incidence of primary brain cancer in New Zealand, 1995 to 2010. Aust NZ J Public Health. 2015;39:148–52.
Karipidis K, Elwood M, Benke G, Sanagou M, Tjong L, Croft RJ. Mobile phone use and incidence of brain tumour histological types, grading or anatomical location: a population-based ecological study. BMJ Open. 2018;8:e024489.
Download references
This work was supported by the Australian Government’s Electromagnetic Energy Program. This work was also partly supported by National Health and Medical Research Council grant no. 1042464.
Author information
Authors and affiliations.
Australian Radiation Protection and Nuclear Safety Agency, Melbourne, VIC, Australia
Ken Karipidis, Rohan Mate, David Urban & Rick Tinker
School of Health Sciences, Swinburne University of Technology, Melbourne, VIC, Australia
- Andrew Wood
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ken Karipidis .
Ethics declarations
Conflict of interest.
The authors declare no competing interest
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Karipidis, K., Mate, R., Urban, D. et al. 5G mobile networks and health—a state-of-the-science review of the research into low-level RF fields above 6 GHz. J Expo Sci Environ Epidemiol 31 , 585–605 (2021). https://doi.org/10.1038/s41370-021-00297-6
Download citation
Received : 30 July 2020
Revised : 23 December 2020
Accepted : 21 January 2021
Published : 16 March 2021
Issue Date : July 2021
DOI : https://doi.org/10.1038/s41370-021-00297-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epidemiology
- Health studies
This article is cited by
Effects of radiofrequency field from 5g communication on fecal microbiome and metabolome profiles in mice.
- Guiqiang Zhou
- Guirong Ding
Scientific Reports (2024)
What evidence exists on the impact of anthropogenic radiofrequency electromagnetic fields on animals and plants in the environment: a systematic map
- Ken Karipidis
- Chris Brzozek
- Andrew W Wood
Environmental Evidence (2023)
Comment on “5G mobile networks and health-a state-of-the-science review of the research into low-level RF fields above 6 GHz” by Karipidis et al.
- Steven Weller
- Igor Belyaev
Journal of Exposure Science & Environmental Epidemiology (2023)
The implications of 5G technology on cardiothoracic surgical services in India
- Aditya Narsipur Doddamane
- Arkalgud Sampath Kumar
Indian Journal of Thoracic and Cardiovascular Surgery (2023)
What evidence exists on the impact of anthropogenic radiofrequency electromagnetic fields on animals and plants in the environment? A systematic map protocol
Environmental Evidence (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Advancements and Challenges in 5G Networks
Ieee account.
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.

IMAGES
VIDEO